Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of central nervous system metastases from non-small cell lung cancer: the present and the future
Introduction
Lung cancer is one of the major causes of cancer related mortality worldwide accounting for approximately 1.4 million deaths per year (1). In approximately 25–40% of non-small cell lung cancer (NSCLC), brain metastases (BM) complicate clinical evolution of disease causing the onset of neurological symptoms, the deterioration in quality of life (QoL) and reducing overall survival (OS) (2,3). About 10–20% of patients (pts) show BM at diagnosis whilst another 20% experience brain progression during the course of disease, often within the first 2 years from diagnosis (2-6). Central nervous system (CNS) represents the first site of relapse after radical treatments for loco-regional disease (7). Furthermore, the prolongation of survival of NSCLC pts, due to the therapeutic advances of the last decades, is likely to explain the increased incidence of BM over time. Unfortunately, for pts with BM the prognosis remains poor with a median OS equal or less than 3 months without any treatment (8). To date, systemic therapy is the standard strategy for metastatic disease. Nevertheless, the blood-brain barrier (BBB) presence with its continuous endothelium, tight junctions, basal membrane, efflux membrane transporters and absence of fenestrations, makes CNS a sanctuary site. Most chemotherapeutic agents do not cross BBB and only the crossing of small lipid-soluble molecules is allowed (9-12). For this reason the role of systemic chemotherapy in the treatment of CNS secondary lesions is controversial (13,14). In the case of macroscopically evident BM, both tumor neoangiogenesis and BBB destruction due to tumor growth, seem to favor intracranial penetration of chemotherapeutic drugs (15,16). This phenomenon could support the use of upfront chemotherapy for BM that damage the integrity of the barrier (15,16). First line upfront platinum based chemotherapy has been evaluated in different prospective trials and an objective response rate (ORR) of 23–50% was reported (5,17-24). Pemetrexed and temozolomide showed some activity (25-29) while 5-FU, topotecan and vinorelbine, did not show any improvement in ORR and OS (23,30,31).
To date local treatments, including whole brain radiation therapy (WBRT), surgery and/or stereotactic radiosurgery (SRS) represent the most used approaches in pts with BM (32). WBRT, in association with corticosteroids, showed a median OS that ranges from 2.4 to 4.8 months (33-35). In some cases, considering the site and the number of lesions, surgery or SRS can be used (32,36-38). Usually SRS is applied when few or small volume isolated lesions (maximum diameter 4 cm) are present (32). WBRT significantly improves brain tumor control after SRS but the role of adjuvant WBRT remains undefined because of the increased risk of neurocognitive toxicity (36). If surgery does not seem useful for multiple BM, prospective trials documented an advantage in terms of survival and local control with surgery and WBRT compared with WBRT alone in oligometastatic brain disease (37,38). Moreover the combination of the three options can be evaluated in selected cases as well as their association with chemotherapy and targeted therapy (32,36-38).
In particular targeted treatments directed against epidermal growth factor receptor (EGFR), such as gefitinib, erlotinib and afatinib, achieved important results in NSCLC, in particular in pts harboring activating EGFR mutations. Considering their favorable safety profile, tyrosine kinase inhibitors (TKIs) may represent a valid alternative in pts with BM but to date the role of TKIs, and their correct place within the therapeutic strategy in this setting, are still debated. Furthermore other new-generation TKIs, such as osimertinib and rociletinib, have already shown important activity on intracranial disease and several trials are still ongoing to evaluate their activity and efficacy.
Here, we review literature data about EGFR-TKIs use in pts with BM from NSCLC, analyzing the most relevant aspects concerning their role and effectiveness compared to current standard treatments.
EGFR mutated NSCLC metastatic to the brain
Approximately 10–15% of NSCLC Caucasian pts show EGFR gene somatic activating mutations (39). Exon 19 in-frame deletion and exon 21 point mutation L858R are the most frequent aberrations, representing about 90% of cases (39). Mutations in EGFR gene cause the expression of a structurally altered receptor that, through the activation of different signaling pathways, promotes cell proliferation and survival (40). In recent years EGFR-TKIs (erlotinib, gefitinib and afatinib) specifically directed against EGFR, and in particular against its mutated form, changed the paradigm of care for a subgroup of NSCLC. Their superiority in terms of efficacy and toxicity in comparison to standard chemotherapy has led to EGFR-TKIs approval for first line treatment of EGFR mutated NSCLC (41-44). Several studies suggested a significant association between EGFR mutation and risk of developing BM, with a reported higher incidence of BM, both at the time of diagnosis and during the course of disease, in EGFR mutated compared with EGFR wild-type (WT) pts (45-48). Generally pts with EGFR mutations had longer OS after BM diagnosis than EGFR WT pts (47,48). However, these data were not confirmed by all studies (49-52).
For this reason more effective agents are needed in order to prolong survival, maintain neurocognitive functions and prevent neurologic deterioration. The high rates of durable response and the good safety profile make EGFR-TKIs an attractive therapeutic option also in these pts, especially considering that standard local approaches in pts metastatic to the brain are associated with a high rate of adverse events (36).
First generation EGFR-TKIs
Erlotinib and gefitinib are reversible TKIs targeting EGFR, the first to enter into clinical practice. Initially, they reported an improvement in progression-free survival (PFS) and OS compared to placebo when used as second line therapy in unselected NSCLC pts, especially never-smokers, females or Asian pts (53,54). Later EGFR mutational status became the most accurate predictor of response to EGFR-TKIs in NSCLC (39,55). Today erlotinib and gefitinib, together with the second generation TKI afatinib, are recognized as the standard first line therapy in NSCLC pts with activating EGFR mutations, instead of conventional cytotoxic chemotherapy. Randomized studies showed that in this setting they were able to obtain an ORR of 60–80%, a PFS ranging from 10 to 13 months and an OS of 13–20 months (41-43,56-65).
CNS penetration
Evidences suggest that EGFR-TKIs can cross the BBB (66,67). Nevertheless, despite their small molecular weight, both erlotinib and gefitinib, seem to reach limited concentrations into cerebrospinal fluid (CSF). In fact, at standard dose CSF levels are lower than plasma levels (68-72). Available data do not favor one EGFR-TKI over another but the concentration and the penetration in CSF are significantly higher with erlotinib than gefitinib (73-75). Moreover, P-glycoprotein (P-gp) efflux pump, that is associated with multiple drug resistance in brain tumor, has gefitinib as one of its substrates (76).
The limited CNS exposure to TKIs can explain the high incidence of BM in EGFR mutated NSCLC despite the good control of extracranial disease during EGFR-TKIs therapy. However, BM occurrence can damage the integrity of BBB and favor TKIs penetration (77). So, while erlotinib and gefitinib at the standard dose do not sufficiently penetrate BBB in absence of CNS involvement, when BM are evident, they probably improve their CNS concentration with a consequent improvement in central activity (67). Furthermore the inadequate TKIs penetration across the intact BBB, could explain the frequent absence of secondary resistance mutations in BM also when they are present in extra-cranial disease sites (70,77,78).
Alternative schedules
Literature data report that dose escalation, pulsate dose or switching TKIs, seem to improve TKIs concentration in CNS and to relieve resistance to standard TKIs treatment in pts with BM from EGFR mutated NSCLC (70-73,76,78-83) (Table 1).
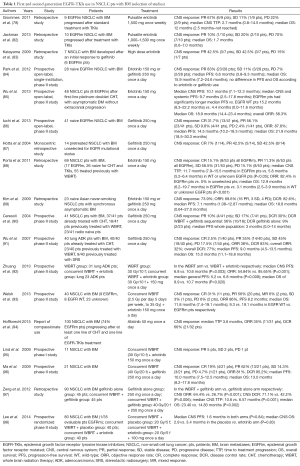
Full table
Progressively increasing doses of erlotinib or gefitinib are able to control BM progression or relapse in NSCLC pts (70-73,79,80). The greater penetration through the BBB when plasma concentrations are higher, also thanks to P-gp saturation, allows EGFR-TKIs to exert greater activity in CNS (76). However, dose escalation is inevitably related to more frequent and significant side effects including high grade fatigue, nausea and liver damage (70-73,79,80).
Pulsate high dose erlotinib, with a median dose of 1,500 mg weekly, appears to provide a significant advantage with reduced toxicity (81). In a small retrospective analysis of nine NSCLC pts, higher pulsate erlotinib dosage (1,500 mg once a week) achieved 67% partial response (PR) after progression to conventional dose (78). In contrast, another retrospective evaluation of ten NSCLC pts who received pulsate dose erlotinib for CNS progression, reported an ORR of 10% with a very limited median OS (1.7 months) (82). To date, there is no prospective trial comparing pulsate high dose vs. standard dose TKIs, but pulsed high doses of EGFR-TKIs could be considered in NSCLC pts with brain progression after standard EGFR-TKI therapy.
Switching to different EGFR-TKIs may represent another valid therapeutic alternative. In a small trial (83), seven lung cancer pts with good response to gefitinib, showed interesting results receiving erlotinib at the time of brain progression: three PR, three stable disease (SD) and one progressive disease (PD) with improvement in PS and neurological symptoms control.
All these results are very preliminary. Further larger prospective studies are needed to validate these approaches in clinical practice.
Standard schedules
Today although their emerging role, the specific indication of EGFR-TKIs in the management of BM from NSCLC, with or without radiotherapy, remains not well defined. Literature data suggest that TKIs alone are able to obtain a high intracranial ORR (99-101) (Table 1). In preclinical mouse model of EGFR mutated NSCLC with BM, gefitinib has proven effective (102). Complete and sustained responses following BM treatment with erlotinib and gefitinib have been reported in several case reports (103-106). Several small phase II trials, have shown that TKIs alone can obtain 75–88% of intracranial ORR in pts with EGFR mutated NSCLC who have not received any prior local therapy for BM (84-86). An open-label, single-institution, phase II study (84) prospectively evaluated the efficacy of EGFR-TKIs, erlotinib or gefitinib, in pts with BM from NSCLC harboring EGFR mutations. Pts did not receive any prior therapy for existing BM. Twenty-three (83%) out of 28 enrolled pts showed PR, 3 (11%) had SD with a disease control rate (DCR) of 93%. Median PFS and OS were 6.6 months (95% CI, 3.8–9.3 months) and 15.9 months (95% CI, 7.2–24.6 months), respectively. There were no differences in PFS and OS between the different TKIs. After progression, 14 pts (50%) received local therapy, either WBRT or SRS, with a local therapy-free interval of 12.6 months (95% CI, 7.6–17.6 months). An Asian phase II, open-label study (85) evaluated the efficacy and safety of erlotinib in NSCLC with BM after first line platinum-based chemotherapy. Forty-eight NSCLC pts with adenocarcinoma histology or activating EGFR mutation and asymptomatic BM, without extra-cranial progression after first-line therapy, were enrolled. The ORR, both intra and extra-cranial, was 58.3%. The median PFS was 10.1 months (95% CI, 7.1–12.3 months) for intracranial progression and 9.7 months (95% CI, 2.5–17.8 months) for both intracranial and systemic progression. Median PFS was significantly longer in pts with EGFR mutated disease than in those with EGFR wild-type disease, 15.2 months (95% CI, 8.3–22.2 months) vs. 4.4 months (95% CI, 0.0–11.6 months; P=0.02), respectively. Most common adverse events were predominantly of grade 1/2. In this trial erlotinib given alone was active and well tolerated also as second line treatment in NSCLC pts with BM. The BM responses to gefitinib, even without irradiation, were reported in a third phase II trial (86) in which 41 pts with BM from EGFR mutated lung adenocarcinoma were enrolled. The ORR was 87.8%, median PFS and OS were 14.5 months (95% CI, 10.2–18.3 months) and 21.9 months (95% CI, 18.5–30.3 months), respectively. Exon 19 deletion was associated with better outcome in both PFS (P=0.003) and OS (P=0.025) compared with L858R. No pts experienced grade ≥4 toxicity.
Several retrospective analyses confirmed the efficacy of TKIs used alone in BM, in particular in EGFR mutated NSCLC (87-89). Gefitinib was evaluated in a Japanese monocentric retrospective study (87) of 57 pts with advanced NSCLC unselected for EGFR mutational status. Fourteen pts had BM. Six of them experienced objective responses to brain lesions [one complete response (CR) and five PR] and eight had SD. Objective responses in extracranial disease were reported in 7 of 14 pts with BM and, interestingly, intracranial objective responses were documented in 6 (86%) of these pts. Porta et al. (88) retrospectively evaluated erlotinib therapy in 69 pts with BM from NSCLC, 17 of whom harboring EGFR activating mutations. Overall ORR in mutated pts was 82.4%, while no responses were observed in unselected ones. The median time to treatment progression (TTP) for intracranial disease in mutated group was 11.7 months (95% CI, 7.9–15.5 months) compared with 5.8 months (95% CI, 5.2–6.4 months) in WT or unknown EGFR pts (P<0.05). The OS was 12.9 vs. 3.1 months in the two groups, respectively (P<0.001). Erlotinib was equally tolerated. Finally, in another retrospective analysis (89), 23 Korean never-smoking pts with lung adenocarcinoma and synchronous asymptomatic BM, treated with either gefitinib or erlotinib as first-line, were considered. They had received no prior treatment, nor chemotherapy nor any kind of radiotherapy. Out of 23 pts, 16 achieved PR, 3 SD and only 4 pts experienced PD, resulting in an ORR of 69.6% and a DCR of 82.6%. Seventeen pts (73.9%) showed intracranial tumor response. The median PFS and OS were 7.1 (95% CI, 1.08–12.87 months) and 18.8 months (95% CI, 0.64–27.0 months), respectively. According with these results clinical benefit from EGFR-TKIs seems to be mainly associated with the presence in the EGFR gene of activating mutations or with those clinical features (sex, ethnicity, smoking status) strongly related to this genotype.
Promising results were also reported in other prospective trials (90,91). In the study by Ceresoli et al. (90) gefitinib was prospectively evaluated in 41 NSCLC pts with BM, of which 37 had already received chemotherapy while 18 had been previously treated with WBRT. Gefitinib proved active in both WBRT-treated and WBRT-naive pts. Four pts (10%) reported PR with an overall DCR of 27%. The median duration of response was 13.5 months. In another prospective study (91) in 40 unselected pts, all previously treated with chemotherapy, gefitinib showed an ORR of 32%, a median PFS of 9.0 months (95% CI, 4.5–13.5 months) and an OS of 15.0 months (95% CI, 11.1–18.8 months).
Recently Soon et al. (107), in a systematic review and meta-analysis of 12 prospective and retrospective studies, compared the effects of brain radiotherapy vs. TKIs alone on intracranial disease, in EGFR mutated NSCLC with BM. In contrast with previous data, this meta-analysis showed an advantage in the 2-year OS for the upfront cranial radiotherapy, either WBRT or SRS, compared with TKIs alone (WBRT: 60%, SRS: 93%, TKIs alone: 45%). Nevertheless radiotherapy did not improve disease response and no significant differences in ORR were documented. In general, cranial irradiation caused a rate of neurological adverse events higher than that reported in studies with TKIs alone (84-86), but lower than that of the concurrent upfront WBRT/TKIs studies (92,93). By limiting the analysis to prospective studies, there was no significant difference in intracranial disease control and survival outcomes between concurrent upfront WBRT plus TKIs and TKIs alone. Thus, considering the high intracranial ORR, consistent with results from other reviews (99-101), TKIs alone may be used upfront before WBRT in those pts with EGFR mutated NSCLC and asymptomatic BM. With a similar strategy the side effects of WBRT may be potentially avoided as long as intracranial disease is well controlled by TKIs alone.
Finally, a pooled analysis of published data (108), including 464 pts from 16 different prospective and retrospective trials, was performed. The primary endpoint was to evaluate the effectiveness of EGFR-TKIs in NSCLC pts with BM, particularly in EGFR mutated ones. Out of 464 enrolled pts, 102 had activating EGFR mutations, while in 362 pts the EGFR mutational status was unknown (unselected group). In this analysis EGFR-TKIs yielded significant results, with an intracranial ORR of 51.8%, a DCR of 75.7%, a median PFS of 7.4 months (95% CI, 4.9–9.9 months) and an OS of 11.9 months (95% CI, 7.7–16.2 months). Better results were reported in the mutated group compared to the unselected one: higher ORR (85.0% vs. 45.1%), a trend of greater benefit in DCR (94.6% vs. 71.3%), longer PFS (12.3 vs. 5.9 months) and OS (16.2 vs. 10.3 months). In 12 of the 16 pooled studies EGFR-TKIs were administered alone, while in four studies they were used in combination with WBRT. Subgroup analysis indicated a greater advantage with WBRT and EGFR-TKIs concurrent administration in unselected pts, with a ORR of 66.2% vs. 45.2% and a DCR of 94.4% vs. 73.1%, respectively.
These studies globally showed EGFR-TKIs promising antitumor activity against both intra and extra-cranial disease in pts with NSCLC, supporting their use as treatment of choice also in pts with CNS asymptomatic metastases. In general, the selection of NSCLC pts based on EGFR mutational status or, as surrogate, demographic features, resulted in greater benefit than in unselected pts. So EGFR-TKIs therapy may be the first treatment option for NSCLC metastatic to the brain in pts harboring activating EGFR mutations. Surely, further studies are warranted.
Second generation EGFR-TKIs
Afatinib is an oral irreversible second-generation EGFR-TKI that acts as a pan-HER inhibitor blocking all members of ErbB family. Analogously to first generation TKIs erlotinib and gefitinib, also afatinib today is approved for the treatment of EGFR mutated TKIs-naive NSCLC pts (109,110). It showed preclinical activity in models with EGFR mutations that confer resistance to EGFR-TKIs (111). Its higher binding affinity and broader target could enhance therapeutic efficacy and delay the development of resistance mutations in EGFR-mutated pts (112). Despite the effectiveness in NSCLC with BM, there are evidences that pts treated with first generation EGFR-TKIs over a period of many months may have an increased risk of developing BM (113). In fact the concentration of TKIs in the CSF seems sufficient to inhibit treatment naive but non-TKIs-resistant cells. Moreover the lower drug concentration could select for resistant clones over time (112,113).
Due to its potency at relatively low concentration, afatinib can be effective in the CSF also in the case of resistance to other TKIs. In preclinical studies, afatinib demonstrated high potency and in vitro, the median inhibitory concentration of afatinib was lower than other EGFR-TKIs (109,110). This suggests that afatinib has the potential to treat BM effectively, despite incomplete BBB penetration. Just before clinical approval, Li et al. (114), reported three cases of EGFR mutated NSCLC with BM in which afatinib, with or without combination with local treatment (WBRT or surgery), showed efficacy as first line therapy. In the LUX-Lung 1 study (115) pts already treated with platinum-based chemotherapy and first generation TKIs were randomized to receive afatinib or placebo. Although no benefit in terms of OS was recorded, afatinib achieved a prolonged PFS in comparison with best supportive care (median PFS 3.3 vs. 1.1 months). In two large randomized trials, LUX-Lung 3 (116) and LUX-Lung 6 (117), afatinib was compared to standard chemotherapy as first line therapy in EGFR mutated NSCLC pts showing a statistically significant advantage in PFS (median PFS 13.6 months). The enrollment of pts with stable BM was allowed in all LUX-Lung studies. In May 2010 the afatinib compassionate use program started with the aim to provide drug access after progression with erlotinib or gefitinib. Recently an efficacy analysis, in pts with BM who were treated with afatinib after chemotherapy and an EGFR-TKI within the compassionate use program, has been published (94). In particular 42% of pts reported PR, 39% SD and only 19% PD. Brain responses were documented in 35% of pts. The safety profile of afatinib reflected that of previous experiences. The most important adverse events were diarrhea, dermatological toxicity, nausea, vomiting, and fatigue. The OS was 9.8 months and TTF did not differ in pts with or without BM. Over 70% of pts with BM had either PR or SD and 76% of pts did not develop new metastases. Considering that in the compassionate use pts received afatinib as third line treatment or greater, these data are very outstanding, especially for pts with BM and for pts who developed resistance to reversible EGFR-TKIs. The observed BM responses provide clinical evidence that afatinib concentration in CSF is sufficient to inhibit tumor growth. The subgroup analysis of LUX-Lung 3 trial (116) have further confirmed the effectiveness of first-line afatinib in CNS metastatic setting, with a median PFS of 11.1 vs. 5.4 months in pts who received afatinib or chemotherapy, respectively [hazard ratio (HR), 0.52; P=0.13].
Radiotherapy and EGFR-TKIs
Different data about the association of TKIs and WBRT exist (Table 1). In a preclinical study a synergistic effect of the combination EGFR-TKIs/radiation therapy has been documented (118). This possible synergism may derive from the radio-sensitizing effect of TKIs and from the damage of BBB created by radiation. In vitro radiation caused increased expression of EGFR and the EGFR blockade, both from gefitinib and erlotinib, enhanced sensitization to radiation in different human carcinoma cell lines and tumor xenografts (118,119). Several trials showed that brain irradiation can cause the opening of BBB, playing an important role in increasing TKIs concentrations in CSF (120-122).
A phase I trial, in which NSCLC pts with BM were enrolled, evaluated the toxicity of WBRT with concurrent and maintenance erlotinib showing that erlotinib was well tolerated and the combination did not cause any significant increase in treatment related toxicity (95). Moreover different phase II studies evaluated the efficacy and toxicity of the concurrent approach (93,96). The phase II trial by Ma et al. (96) studied the concomitant treatment with WBRT and gefitinib in 21 Chinese pts with BM from NSCLC to assess its impact on pts QoL and post-treatment survival. All pts received 40 Gy WBRT in 20 fractions. Gefitinib was administered during the radiation course and was continued until progression or unacceptable toxicity. Four (19%) pts had CR, 13 pts showed (62%) PR, 3 pts had SD and only 1 pt showed PD. The ORR was 81%. Median PFS and OS were 10.0 months (95% CI, 7.5–12.5 months) and 13.0 months (95% CI, 8.2–17.8 months), respectively. The great majority of toxicities were grade 2 and QoL was significantly improved following treatment. Erlotinib achieved similar results in a single-arm phase II trial (93) in which 40 NSCLC pts with BM, not selected for EGFR mutations, were treated with standard dose TKIs and concurrent WBRT. The ORR was 86%, median OS was 11.8 months (95% CI, 7.4–19.1 months) and the combination resulted well tolerated with no grade 4 toxicity, limited neurotoxicity and only 3 cases of grade 3 rash (3%). EGFR status was known in 17 pts and median OS was 9.3 vs. 19.1 months in EGFR WT vs. mutated pts respectively. These data are promising and concomitant treatment was well tolerated, with important activity and improvement in QoL.
Concomitant therapy was also compared both to EGFR-TKI alone and WBRT alone (90,92,97,98,123). Ceresoli et al. (90), in a previously mentioned study, evaluated 41 NSCLC pts with BM. Eighteen pts received gefitinib after previous WBRT, 23 pts were radio-naive and 37 pts received previous chemotherapy. Four PR (10%) were observed, SD was reported in seven cases and nearly 30% of pts achieved DCR, showing an interesting activity of gefitinib both in previously irradiated and non-irradiated pts. The median PFS of the whole population was 3 months (95% CI, 0.0–14 months). Neurological improvement was also observed in four of nine symptomatic pts. Combination treatment showed a significant prognostic advantage at the univariate analysis (P=0.0006) obtaining disease control in 10/18 pts (56%) compared to 2/23 (9%) in radio naive pts. These data were confirmed by a retrospective analysis (97) that compared the efficacy of gefitinib alone with gefitinib plus concomitant WBRT. Ninety pts were divided in two groups: the gefitinib group and the gefitinib-WBRT group. The combination group showed higher ORR (64.4% vs. 26.7%, P<0.001) and higher DCR (71.1% vs. 42.2%, P=0.006) with nearly doubled median PFS and OS (10.6 vs. 6.57 months, P<0.001 and 23.40 vs. 14.83 months, P=0.02, respectively). In a recent randomized phase II trial (98) concurrent WBRT and erlotinib compared to WBRT alone failed to demonstrate any advantage in intracranial disease control. The 80 enrolled NSCLC pts metastatic to the brain were predominantly EGFR WT (only 1/35 evaluable pts was mutated). Median PFS was 1.6 months in both arms and median OS was 2.9 and 3.4 months in the placebo compared with erlotinib arm respectively (HR, 0.95; 95% CI, 0.58–1.55; P=0.83). The Radiation Therapy Oncology Group (RTOG) designed a phase III study (123) to test if erlotinib and temozolomide in association to WBRT and SRS could improve OS in NSCLC pts with one to three BM and unknown EGFR mutational status. Unfortunately the combination showed higher percentage of grade 3–5 toxicities without any statistically significant efficacy result and the study was closed early for poor accrual. Finally in another previously cited study (92), 54 NSCLC pts with multiple BM, receiving WBRT with or without concurrent erlotinib, reported an advantage with additional erlotinib regardless of EGFR-mutational status. The ORR was 54.84% vs. 95.65% (P=0.001), with a median brain PFS of 6.8 vs. 10.6 months (P=0.003), a median general PFS of 5.2 vs. 6.8 months (P=0.009) and a median OS of 8.9 vs. 10.7 months (P=0.020) in the WBRT arm and the concurrent arm, respectively. Furthermore erlotinib resulted the most important prognostic factor for prolonged survival at the multivariate analysis. In contrast with literature data, in the combination group there were no differences in brain PFS, general PFS and OS between EGFR-mutated and EGFR WT pts. Thus the EGFR-TKIs radiosensitizing effect in this trial doesn’t seem to be dependent on EGFR-mutations. Nevertheless, in the management of BM, the addiction of TKIs to WBRT as radiosensitizing agents also in WT NSCLC pts, should be confirmed by other specific studies.
To date no prospective study exists that has really compared the use of cranial irradiation alone vs. TKIs alone vs. combination of the two modalities.
Third generation EGFR-TKIs
Although NSCLC pts harboring EGFR sensitizing mutations derive significant clinical advantage from EGFR-TKIs therapy, invariably, after about 9–13 months from the beginning of treatment, disease progression occurs. Several mechanisms of acquired resistance exist: the onset of secondary mutations in EGFR (50–60%), the activation of alternative pathways (1–25%) and the histologic transformation (5–10%). In the remaining 20–30% of cases resistance mechanisms are not known yet (124,125). Surely, the development of EGFR T790M mutation is the most common cause of acquired resistance. The substitution of methionine with threonine at position 790 in the exon 20 blocks the binding of first generation EGFR-TKIs to the ATP pocket and increases its affinity to ATP rather than to EGFR-TKIs (126,127). Third generation EGFR-TKIs (osimertinib, rociletinib, HM61713 and others) have been developed as T790M mutant-specific inhibitors. First data support their effectiveness and safety also in NSCLC pts with BM.
AZD9291 (osimertinib), a novel TKI that specifically and irreversibly binds the cysteine-797 residue in the ATP binding site of EGFR, has recently obtained the accelerated Food and Drugs Administration (FDA) approval in EGFR mutated NSCLC with documented T790M resistance mutation, on the basis of important results of phase I and II trials (128-130). Its activity has been also evaluated in pts with BM from NSCLC. A combined analysis of AURA and AURA 2 (131) studies reported that 39% of enrolled pts (162 of 411 pts) had BM. The systemic ORR of overall population was 61%, and it became 56% and 64% in pts with or without BM respectively. Cases of shrinkage of brain lesions were reported. Currently the Real World Treatment Study of AZD9291 for Advanced/Metastatic EGFR T790M Mutation NSCLC (ASTRIS) is ongoing, to assess the efficacy and safety of single agent AZD9291 in a real world setting in EGFR T790M mutation-positive NSCLC, who have received prior EGFR-TKIs therapy. Also pts with stable BM can be enrolled.
CO1886 (rociletinib) is another irreversible third generation mutant selective EGFR-TKI, specifically directed against common sensitizing EGFR mutations and T790M (132,133). Also rociletinib showed to be effective in BM from NSCLC. Out of 401 pts who received rociletinib within clinical trials 42% (170 of 401 pts) had BM. At the interim analysis pts with BM reached an ORR of 41% (134). At an indirect comparison the ORR of NSCLC pts with or without BM resulted equal to 45% and 55%, respectively (135).
AZD3759 is the first EGFR-TKI designed to penetrate BBB and to achieve high free drug exposure inside the brain, CSF and plasma, with the aim to treat BM and leptomeningeal disease in pts with EGFR mutated NSCLC. In a recent phase I, open-label, multicentre study (136), in pts with advanced stage EGFR mutated NSCLC who progressed after at least one EGFR-TKI and one line of chemotherapy, AZD3759 was well tolerated, achieved sufficient CNS concentration and showed promising antitumor activity in the dose escalation phase. Among 20 pts with measurable BM, 8 had tumor shrinkage in the brain, with 3 confirmed and 3 un-confirmed PR. The most common adverse events were skin rash and diarrhea.
Results of activity in BM of other third generation TKIs, such as ASP8273, EGF816 and HM61713 are still awaited.
Conclusions
First and second generation EGFR-TKIs represent a valid therapeutic option in NSCLC pts with BM (Table 1), especially in pts with activating EGFR mutations. In many studies they are able to obtain similar activity to local treatments, with a beneficial toxicity profile. Probably EGFR-TKIs effectiveness is conditioned by the heterogeneity of the EGFR mutational status between CNS metastases and extracranial disease. Thus, their combination with other treatment options, such as surgery, radiotherapy, chemotherapy, monoclonal antibodies and immunotherapy, may further improve results. The use of biopsy at the time of progression should be always evaluated. Considering the inevitable development of drug resistance, the identification of third generation EGFR-TKIs, able to overcome secondary resistance, is of major importance and is very promising especially in pts with BM. At the same time prospective studies focused on the use of TKIs with or without concurrent WBRT in pts specifically selected on the basis of the EGFR mutational status are needed.
Acknowledgements
The authors want to thank Anna Leone for her valuable support.
Footnote
Conflicts of Interest: MC Garassino declares consultancies from AstraZeneca, Roche, Boehringer. The other authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Center Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Namba Y, Kijima T, Yokota S, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: review of 15 clinical cases. Clin Lung Cancer 2004;6:123-8. [Crossref] [PubMed]
- Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenterphase II trial (GFPC 07-01). Ann Oncol 2011;22:2466-70. [Crossref] [PubMed]
- D'Antonio C, Passaro A, Gori B, et al. Bone and brain metastases in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 2014;6:101-14. [Crossref] [PubMed]
- Markesbery WR, Brooks WH, Gupta GD, et al. Treatment for patients with cerebral metastases. Arch Neurol 1978;35:754-6. [Crossref] [PubMed]
- Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996;78:1781-8. [Crossref] [PubMed]
- Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 2004;10:3728-36. [Crossref] [PubMed]
- Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med 1975;293:161-6. [Crossref] [PubMed]
- Sung C, Blaney SM, Cole DE, et al. A pharmacokinetic model of topotecan clearance from plasma and cerebrospinal fluid. Cancer Res 1994;54:5118-22. [PubMed]
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13-25. [Crossref] [PubMed]
- Moscetti L, Nelli F, Felici A, et al. Up-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: survey by Outcome Research Network for Evaluation of Treatment Results in Oncology. Cancer 2007;109:274-81. [Crossref] [PubMed]
- Postmus PE, Smit EF. Chemotherapy for brain metastases of lung cancer: a review. Ann Oncol 1999;10:753-9. [Crossref] [PubMed]
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999;284:1994-8. [Crossref] [PubMed]
- Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brainmetastases. Lancet Oncol 2002;3:53-7. [Crossref] [PubMed]
- Cortes J, Rodriguez J, Aramendia JM, et al. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology 2003;64:28-35. [Crossref] [PubMed]
- Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer 1998;20:93-8. [Crossref] [PubMed]
- Fujita A, Fukuoka S, Takabatake H, et al. Combination chemotherapy of cisplatin, ifosfamide, andirinotecan with rhG-CSF support in patients with brain metastases fromnon-small cell lung cancer. Oncology 2000;59:291-5. [Crossref] [PubMed]
- Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 1999;85:1599-605. [Crossref] [PubMed]
- Cotto C, Berille J, Souquet PJ, et al. A phase II trial of fotemustine and cisplatin in central nervous system metastases from non-small cell lung cancer. Eur J Cancer 1996;32A:69-71. [Crossref] [PubMed]
- Bernardo G, Cuzzoni Q, Strada MR, et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest 2002;20:293-302. [Crossref] [PubMed]
- Robinet G, Thomas P, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95-1. Ann Oncol 2001;12:59-67. [Crossref] [PubMed]
- Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol 2012;14:491-5. [Crossref] [PubMed]
- Bearz A, Garassino M, Tiseo M, et al. Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer 2010;68:264-8. [Crossref] [PubMed]
- Abrey LE, Olson JD, Raizer JJ, et al. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 2001;53:259-65. [Crossref] [PubMed]
- Christodoulou C, Bafaloukos D, Kosmidis P, et al. Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol 2001;12:249-54. [Crossref] [PubMed]
- Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys 2005;61:185-91. [Crossref] [PubMed]
- Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 2002;20:3644-50. [Crossref] [PubMed]
- Guerrieri M, Wong K, Ryan G, et al. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer 2004;46:107-11. [Crossref] [PubMed]
- Neuhaus T, Ko Y, Muller RP, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer 2009;100:291-7. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Knisely JP, Berkey B, Chakravarti A, et al. A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118). Int J Radiat Oncol Biol Phys 2008;71:79-86. [Crossref] [PubMed]
- Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastases. J Clin Oncol 2006;24:1295-304. [Crossref] [PubMed]
- Priestman TJ, Dunn J, Brada M, et al. Final results of the Royal College of Radiologists trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996;8:308-15. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Tsao MN, Rades K, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastases(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features sociated with epidermal growth factorreceptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for Europeanpatients with advanced EGFR mutation-positive non-small-cell lung cancer(EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [Crossref] [PubMed]
- Han G, Bi J, Tan W, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016. [Epub ahead of print]. [PubMed]
- Stanic K, Zwitter M, Hitiji NT, et al. Brain metastasis in lung adenocarcinoma: impact of EGFR mutations status on incidence and survival. Radiol Oncol 2014;48:173-83. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer 2014;84:86-91. [Crossref] [PubMed]
- Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol 2015;124:79-85. [Crossref] [PubMed]
- Luo D, Ye X, Hu Z, et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol 2014;35:2437-44. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo controlled, multicentre study (iressa survival evaluation in lung cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Schneider CP, Heigener D, Schott-von-Römer K, et al. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the TRUST study. J Thorac Oncol 2008;3:1446-53. [Crossref] [PubMed]
- Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small cell lung cancer. J Natl Cancer Inst 2005;97:643-55. [Crossref] [PubMed]
- Cortes-Funes H, Gomez C, Rosell R, et al. Epidermal growth factor receptor activating mutations in Spanish gefitinitreated non-small-cell lung cancer patients. Ann Oncol 2005;16:1081-6. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [Crossref] [PubMed]
- Han SW, Kim TY, Jeon YK, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res 2006;12:2538-44. [Crossref] [PubMed]
- Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res 2005;11:5878-85. [Crossref] [PubMed]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267-73. [Crossref] [PubMed]
- Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744-52. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080-7. [Crossref] [PubMed]
- Broniscer A, Panetta JC, O’Shaughnessy M, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI–420. Clin Cancer Res 2007;13:1511-15. [Crossref] [PubMed]
- Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013;14:188-93. [Crossref] [PubMed]
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902-8. [Crossref] [PubMed]
- Yang J, Cheng Y, Zhao M, et al. A phase II trial comparing pemetrexed with gefitinib as the second-line treatment of nonsquamous NSCLC patients with wild-type EGFR (CTONG0806). J Clin Oncol 2013;31:abstr 8042.
- Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99:283-6. [Crossref] [PubMed]
- Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small cell lung cancer. Mol Clin Oncol 2014;2:116-20. [PubMed]
- Hata A, Kaji R, Fujita S, et al. High-dose erlotinib for refractory brain metastases in a patient with relapsed non-small cell lung cancer. J Thorac Oncol 2011;6:653-4. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Wang M, Jing Z, Minjiang C, et al. Cerebral penetration of gefitinib in patients with lung adenocarcinoma. J Clin Oncol 2011;29:abstr 7608.
- Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74. [Crossref] [PubMed]
- Chen Y, Wang M, Zhong W, et al. Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer 2013;82:313-8. [Crossref] [PubMed]
- Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01–03 and 00–01. Clin Cancer Res 2005;11:7841-50. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. ‘‘Pulsatile’’ high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 2006;24:4517-20. [Crossref] [PubMed]
- Togashi Y, Masago K, Fukudo MY, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol 2011;68:1089-92. [Crossref] [PubMed]
- Milton DT, Azzoli CG, Heelan RT, et al. A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer 2006;107:1034-41. [Crossref] [PubMed]
- Jackman DM, Mach SL, Heng JC. Pulsed dosing of erlotinib for central nervous system (CNS) progression in EGFR-mutant non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8116.
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harbouring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second line treatment in patients with advanced non-small cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol 2013;24:993-9. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Hotta K, Kiura K, Ueoka H, et al. Effect of gefitinib (`Iressa', ZD1839) on brain metastases in patients withadvanced non-small-cell lung cancer. Lung Cancer 2004;46:255-61. [Crossref] [PubMed]
- Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [Crossref] [PubMed]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351-4. [Crossref] [PubMed]
- Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004;15:1042-7. [Crossref] [PubMed]
- Wu C, Li YL, Wang ZM, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;57:359-64. [Crossref] [PubMed]
- Zhuang H, Yuan Z, Wang J, et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther 2013;7:1179-86. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156-63. [Crossref] [PubMed]
- Lind JS, Lagerwaard FJ, Smit EF, et al. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;74:1391-6. [Crossref] [PubMed]
- Ma S, Xu Y, Deng Q, et al. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer 2009;65:198-203. [Crossref] [PubMed]
- Zeng YD, Zhang L, Liao H, et al. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non-small-cell lung cancer: a retrospective study. Asian Pac J Cancer Prev 2012;13:909-14. [Crossref] [PubMed]
- Lee SM, Lewanski CR, Counsell N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst 2014;106:dju151. [Crossref] [PubMed]
- Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res 2012;18:938-44. [Crossref] [PubMed]
- Bartolotti M, Franceschi E, Brandes AA. EGF receptor tyrosine kinase inhibitors in the treatment of brain metastases from non-small cell lung cancer. Expert Rev Anticancer Ther 2012;12:1429-35. [Crossref] [PubMed]
- Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 2014;40:716-22. [Crossref] [PubMed]
- Heimberger AB, Learn CA, Archer GE, et al. Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR tyrosine kinase inhibitor ZD1839 (iressa). Clin Cancer Res 2002;8:3496-502. [PubMed]
- Popat S, Hughes S, Papadopoulos P, et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer 2007;56:135-7. [Crossref] [PubMed]
- Lai CS, Boshoff C, Falzon ML, et al. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax 2006;61:91. [Crossref] [PubMed]
- Fekrazad MH, Ravindranathan M, Jones DV Jr. Response of intracranial metastases to erlotinib therapy. J Clin Oncol 2007;25:5024-6. [Crossref] [PubMed]
- Gounant V, Wislez M, Poulot V, et al. Subsequent brain metastasis responses to epidermal growth factor receptor tyrosine kinase inhibitors in a patient with non-small-cell lung cancer. Lung Cancer 2007;58:425-8. [Crossref] [PubMed]
- Soon YY, Leong CN, Koh WE, et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: A systematic review and meta-analysis. Radiother Oncol 2015;114:167-72. [Crossref] [PubMed]
- Fan Y, Xu X, Xie C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: a pooled analysis of published data. Onco Targets Ther 2014;7:2075-84. [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342-50. [Crossref] [PubMed]
- Lynch TJ, Adjei AA, Bunn PA Jr, et al. Summary statement: novel agents in the treatment of lung cancer: advances in epidermal growth factor receptor-targeted agents. Clin Cancer Res 2006;12:4365s-4371s. [Crossref] [PubMed]
- Druker BJ. Circumventing resistance to kinase-inhibitor therapy. N Engl J Med 2006;354:2594-6. [Crossref] [PubMed]
- Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 2010;116:1336-43. [Crossref] [PubMed]
- Li SH, Hsieh MH, Fang YF. Afatinib in Treatment-Naive Patients With EGFR-Mutated Lung Adenocarcinoma With Brain Metastasis A Case Series. Medicine (Baltimore) 2015;94:e1739. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3342-50. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328-35. [PubMed]
- Akimoto T, Hunter NR, Buchmiller L, et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res 1999;5:2884-90. [PubMed]
- Qin D, Ma J, Xiao J, et al. Effect of brain irradiation on blood-CSF barrier permeability of chemotherapeutic agents. Am J Clin Oncol 1997;20:263-5. [Crossref] [PubMed]
- Qin D, Ou G, Mo H, et al. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier: clinical results. Int J Radiat Oncol Biol Phys 2001;51:959-62. [Crossref] [PubMed]
- DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989;39:789-96. [Crossref] [PubMed]
- Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgeryalone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys 2013;85:1312-8. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing theaffinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Ramalingam S, Yang JC, Lee CK, et al. Osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two Phase I expansion cohorts. J Thorac Oncol 2016;11:S152. [Crossref] [PubMed]
- Yang J, Ramalingam SS, Jänne PA, et al. LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol 2016;11:S152-3. [Crossref] [PubMed]
- Kim D, Yang J, Cross D, et al. Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases (BM). Ann Oncol 2014;25:iv146-64.
- Ahn MJ, Tsai CM, Yang JC, et al. 3083 AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from Phase II studies. Eur J Cancer 2015;51:S625-6. [Crossref]
- Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M mediated resistance in NSCLC. Cancer Discov 2013;3:1404-15. [Crossref] [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non–small-cell lung cancer. N Engl J Med 2015;372:1700-9. [Crossref] [PubMed]
- Camidge DR, Sequist LV, Soria JC, et al. Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement. J Thorac Oncol 2015;10:S319.
- Varga A, Camidge DR, Sequist LV, et al. Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement. Eur J Cancer 2015;51:S598. [Crossref]
- Ahn MJ, Kim DW, Kim TM, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol 2016;34:abstr 9003.


