ASCEND-2: a canary in a coal mine for descending to second-line treatment for ALK-rearranged non-small cell lung cancer
The discovery of oncogenic driver mutations has revolutionized the management of non-small cell lung cancer (NSCLC) for the past decade. The prevalence of anaplastic lymphoma kinase (ALK) rearrangement in NSCLC is estimated to be 2–7% (1). Crizotinib, a tyrosine kinase inhibitor (TKI), which targets ALK as well as ROS1 and c-MET, was approved by the U.S. Food and Drug Administration (FDA) in 2011 for the treatment of metastatic ALK-rearranged NSCLC. Several studies have demonstrated a median progression-free survival (PFS) of approximately 8 to 11 months and response rates of 65% to 75% (2,3). However, as with other targeted therapies, patients eventually progress due to the emergence of acquired resistance via the activation or inhibition of alternative signaling pathways, secondary ALK mutations, or amplification of the ALK fusion gene.
Disease progression is often seen either in the brain or liver. One retrospective analysis showed that 41% of patients with ALK-rearranged and crizotinib-resistant NSCLC developed brain metastases while 25% had liver involvement (4). Another study reported that among patients without baseline brain metastases who received crizotinib after failing chemotherapy, 20% progressed with evidence of new brain lesions (5). These are largely due to the fact that crizotinib has limited penetration of the blood-brain barrier. Therefore, it is of paramount importance to develop new treatment options for patients who have failed crizotinib; particularly a targeted compound with activity in the brain is highly desirable.
Ceritinib is an oral selective TKI of ALK that is 20 times more potent than crizotinib in enzymatic assays (6). Unlike crizotinib, ceritinib does not inhibit the kinase activity of c-MET but targets ROS1 and insulin-like growth factor 1 receptor. A phase 1 study of ceritinib in 130 patients with locally advanced or metastatic ALK-rearranged cancers was reported by Shaw et al. (ASCEND-1) (7). A total of 59 patients in the dose-escalation phase were given ceritinib at 50 to 750 mg once a day. The dose-limiting toxicities included diarrhea, vomiting, dehydration, elevated aminotransferase levels, and hypophosphatemia. In the expansion phase, another 71 patients were given ceritinib at 750 mg once a day. The majority of patients had NSCLC; overall response rate (ORR) was 58% [95% confidence interval (CI), 48–67] and median PFS was 7 months (95% CI, 5.6–9.5). Following the publication of ASCEND-1, ceritinib was granted accelerated approval by the U.S. FDA in April 2014 for the treatment of ALK-rearranged metastatic NSCLC after progression or intolerance of crizotinib. The updated analysis of ASCEND-1 included a larger cohort of patients with ALK-rearranged NSCLC treated with ceritinib at 750 mg once a day, of whom 163 were ALK inhibitor-pretreated and 83 were ALK inhibitor-naive with ORR of 56% (95% CI, 49–64) and 72% (95% CI, 61–82) respectively. Median duration of response (DOR) was 8.3 months (95% CI, 6.8–9.7) for ALK inhibitor-pretreated patients; median PFS was 6.9 months (95% CI, 5.6–8.7); and median overall survival (OS) was 16.7 months [95% CI, 14.8–non-estimable (NE)]. In contrast, median DOR for ALK inhibitor-naive patients was 17 months (95% CI, 11.3–NE); median PFS was 18.4 months (95% CI, 11.1–NE); and median OS had not been reached (95% CI, 19.6–NE) (8).
In the article accompanying this editorial, Crinò et al. reported a single-arm, open-label, multicenter, phase 2 study of ceritinib in a heavily pretreated patient population with ALK-rearranged NSCLC (ASCEND-2) (9). Before starting ceritinib, patients must have received at least two lines of therapy including platinum-based chemotherapy and crizotinib. Prior exposure to ALK inhibitors other than crizotinib was excluded and crizotinib had to be the last line of systemic treatment. Efficacy and safety data collected from 140 enrolled patients were published at the Journal of Clinical Oncology in August 2016. Investigator-assessed ORR, the trial’s primary end point, was 38.6% (95% CI, 30.5–47.2). Disease control rate (DCR) was 77.1% (95% CI, 69.3–83.8); median time to response was 1.8 months (95% CI, 1.6–5.6); median DOR was 9.7 months (95% CI, 7.1–11.1); and median PFS was 5.7 months (95% CI, 5.4–7.6). A pre-planned subgroup analysis of whole-body response in 100 patients with baseline brain metastases was also reported. Investigator-assessed ORR was 33% (95% CI, 23.9–43.1); DCR was 74% (95% CI, 64.3–82.3%); median DOR was 9.2 months (95% CI, 5.5–11.1); and median PFS was 5.4 months (95% CI, 4.7–7.2). Only 20 out of the above 100 patients had active brain metastases at baseline. They achieved 45% (95% CI, 23.1–68.5) intracranial ORR and 80% (95% CI, 56.3–94.3) intracranial DCR.
Toxicities were not negligible. All patients had at least one reported adverse event (AE) and the most prevalent ones were: nausea (81.4%), diarrhea (80%), and vomiting (62.9%). A total of 71.4% of patients experienced grade 3 or 4 AEs including elevated alanine aminotransferase (15.7%) and γ-glutamyltransferase (9.3%) levels. Serious AEs were reported in 40.7% of patients. One patient developed grade 4 pneumonitis and one patient developed grade 3 QTc prolongation. Hyperglycemia and bradycardia were also observed. Dose interruptions for at least 1 day were required in 75.7% of patients, of which 85.8% were due to AEs. At least 1 dose reduction occurred in 54.3% of patients, of which 84.2% were attributable to AEs.
A confirmatory phase 3 ASCEND-5 study enrolled similar patient population as ASCEND-2 and results were recently presented at the European Society for Medical Oncology 2016 congress (10). A total of 231 patients pretreated with one or two chemotherapy regimens and crizotinib for locally advanced or metastatic ALK-positive NSCLC were randomized to ceritinib or chemotherapy. Median PFS by blinded independent review committee (BIRC) was 5.4 months (95% CI, 4.1–6.9) for ceritinib and 1.6 months for chemotherapy (95% CI, 1.4–2.8) with hazard ratio (HR) of 0.49 (95% CI, 0.36–0.67; P<0.001). ORR was 39.1% (95% CI, 30.2–48.7) and 6.9% (95% CI, 3–13.1) respectively. The ceritinib arm approximates ASCEND-2 in terms of median PFS (5.4 versus 5.7 months) and ORR (39.1% versus 38.6%), but disappointingly the chemotherapy arm only had median PFS of 1.6 months. Although the trial population was heavily pretreated, one would have hoped for a better outcome. Patients on ASCEND-2 had to fail at least two lines of chemotherapy while over 85% of the patients on ASCEND-5 only received one line. The further decline of median PFS from ASCEND-2 to ASCEND-5 was completely underwhelming.
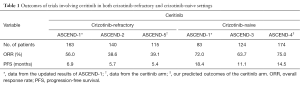
How about the efficacy of ceritinib in the crizotinib-naive patient population? The ASCEND-1 data were quite impressive with median PFS of 18.4 months. However, only 83 patients were enrolled in that arm. ASCEND-3, a phase 2 study of ceritinib in ALK inhibitor-naive patients with ALK-rearranged NSCLC was presented at the 2015 American Society of Clinical Oncology (ASCO) annual meeting (11). Among 124 patients, whole body ORR was 63.7% (95% CI, 54.6–72.2); whole body DCR was 89.5% (95% CI, 82.7–94.3); median DOR was 9.3 months (95% CI, 9.1–NE); and median PFS was 11.1 months (9.3 to NE). ASCEND-4, which compares ceritinib to chemotherapy in previously untreated patients, is ongoing. Both ASCEND-3 and PROFILE 1007 evaluated ALK inhibitor-naive patients. While PROFILE 1007 required failing of one prior platinum-based regimen, ASCEND-3 permitted ≤3 lines of chemotherapy. The crizotinib arm in PROFILE 1007 showed ORR of 65% and median PFS of 7.7 months (2). In comparison, ASCEND-3 did not demonstrate significantly better outcomes with ceritinib. Similar to ASCEND-4, PROFILE 1014 enrolled patients without any previous systemic treatment. ORR was 74% and median PFS was 10.9 months for patients randomized to crizotinib (3). Using PROFILE 1007 and PROFILE 1014 as a guide with an increment of ORR by approximately 10% and median PFS by 3.2 months, one may speculate that the ceritinib arm in ASCEND-4 would show ORR of roughly 75% and median PFS of 14.5 months (Table 1).
Full table
So what is the significance of ASCEND-2 in the whole development of ceritinib? The answer is not much if any. Is there a role for ceritinib going forward, particularly in the context of other next-generation ALK inhibitors such as alectinib and brigatinib?
Alectinib is a potent and highly selective TKI of ALK with activity against both wide-type and mutated ALK including the L1196M gatekeeper mutation that confers resistance to crizotinib (12). In comparison to crizotinib, alectinib also inhibits RET but not ROS1 or c-MET. It has already received breakthrough therapy designation by the U.S. FDA for first-line treatment of patients with ALK-positive NSCLC based on the J-ALEX study presented at the 2016 ASCO annual meeting (13). A total of 207 patients who had received no more than one line of chemotherapy were randomized to alectinib at 300 mg twice a day versus crizotinib. ORR in the intention to treat population assessed by BIRC was 76.7% (95% CI, 68.5–84.9) for alectinib and 68.9% (95% CI, 60–77.9) for crizotinib. Median PFS was not reached (NR) (95% CI, 20.3–NR) and 10.2 months (95% CI, 8.2–12) respectively with HR of 0.34 (99.6826% CI, 0.17–0.71; P<0.0001). These outcomes were very impressive although questions arose as to the fact that there were fewer patients with brain metastases on the alectinib arm (13.6% versus 27.9%). The subgroup analysis of patients with brain metastases significantly favored alectinib with HR of 0.08 (95% CI, 0.01–0.61), which could not be explained by the presenter. We are eager to see whether the global ALEX trial results will be further convincing for alectinib to replace crizotinib in the front-line setting. Nevertheless, compared to J-ALEX, the results of ASCEND-3 were far inferior. If our prediction for ASCEND-4 holds true, ceritinib will never achieve front-line status.
Brigatinib is another next-generation ALK inhibitor that is pending U.S. FDA review for accelerated approval in ALK-rearranged and crizotinib-resistant NSCLC. The ALTA trial, a randomized phase 2 study of brigatinib at 90 mg once a day versus 90 mg once a day for 7 days followed by 180 mg once a day in patients with crizotinib-resistant ALK-positive NSCLC, was also presented at the 2016 ASCO annual meeting (14). ORR by investigator assessment was 45% (95% CI, 34–56) for 112 patients enrolled in the lower dose arm and 54% (95% CI, 43–65) for 110 patients enrolled in the higher dose arm. Median PFS was 9.2 (95% CI, 7.4–15.6) and 12.9 (95% CI, 11.1–NR) months respectively with HR of 0.55 (95% CI, 0.35–0.86). Median OS was NR in either arm. In terms of AEs, most were grade 1 or 2 and some appeared dose-related. The most grade 3 or above AEs seen with brigatinib at 180 mg once a day included increased serum creatine phosphokinase (9%), hypertension (6%), rash (3%), dyspnea (2%), and back pain (2%). Early-onset pulmonary AEs defined as dyspnea, hypoxia, cough, pneumonia, or pneumonitis occurred in 6% of patients, all of which occurred while patients were on brigatinib at 90 mg once a day and did not increase when the dose was escalated to 180 mg once a day. It was suggested that <7 days of crizotinib washout might result in more incidences than ≥7 days with HR of 2.52 (95% CI, 0.82–7.8). Currently a phase 3 study of brigatinib at 180 mg once a day versus crizotinib in ALK inhibitor-naive patients is recruiting participants (ALTA-1L). It would be interesting to see if brigatinib can achieve the same magnitude of efficacy as alectinib in the front-line setting.
Although there are no randomized head-to-head trials to directly compare next-generation ALK inhibitors, it seems clear that ceritinib did not outperform either alectinib or brigatinib in terms of efficacy or safety. For example, in the second-line setting phase 2 studies showed an ORR of 38.6%, 49%, and 54% for ceritinib, alectinib, and brigatinib (higher dose arm). Median PFS was 5.7, 8.9, and 12.9 months respectively (9,14,15). All of them demonstrated far superior CNS activities than crizotinib with intracranial ORR of 33%, 42.6%, and 30.6% for ceritinib, alectinib, and brigatinib among patients with both measurable and non-measureable brain metastases (9,14,16). Ceritinib was the least tolerated TKI as it caused the most gastrointestinal AEs. For instance, nausea of any grades was reported to be 81.4%, 12%, and 40% for ceritinib, alectinib, and brigatinib (9,14,15). Pneumonitis is an often feared AE related to TKIs. The frequency of pneumonitis of any grade was 1.4% for ceritinib and 0% for alectinib (9,15). With brigatinib, early-onset pulmonary events in ATLA were 6% (14).
In conclusion, even though ceritinib is a treatment option for patients with ALK-rearranged NSCLC who have progressed on or are intolerant of crizotinib, the significance of this indication is trivial. The rapid descend of median PFS from ASCEND-1 to ASCEND-3 has led us to speculate that the outcomes of ASCEND-4 would be underwhelming. In contrast to alectinib which will likely be approved for front-line therapy, it is almost impossible for ceritinib to achieve front-line status. Brigatinib still holds promise unless ALTA-1L turns out to far inferior to J-ALEX. Therefore in the context of the life-cycle of ceritinib, ASCEND-2 is like a canary in a coal mine that foretells a losing battle for ceritinib among next-generation ALK inhibitors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675-90. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Scagliotti G, Kim TM, Crinò L, et al. Ceritinib vs chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with CT and crizotinib (CRZ): results from the confirmatory phase 3 ASCEND-5 study. Ann Oncol 2016;27:vi552-vi587.
- Felip E, Orlov S, Park K, et al. ASCEND-3: A single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naive adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8060.
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): first report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). J Clin Oncol 2016;34:abstr 9007.
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:4079-85. [PubMed]


