Defining the endpoints: how to measure the efficacy of drugs that are active against central nervous system metastases
Brain metastases (BMs) from solid tumors: one biology, one drug and a specific activity
The survival of patients following the development of brain metastasis (BM) is measured in weeks to months, even if a considerable variability is observed in relation to the histotype, the size, the number and site of the brain spreading, as well as the performance status of single patient, the symptoms and the burden of disease. Whereas the overall 2-year survival rate in patients with BM is 8.1%, 2-year survival after diagnosis of BM is less than 2% in patients with SCLC, but as high as 24% in patients with ovarian cancer (1). The findings from the last 5 years of patients with oligometastases to the brain treated primarily with local surgical or radiation therapy reveal a more encouraging median overall survival of 16 months from the time of BM diagnosis (2). Despite the evolution of biomolecular diagnostics, the spreading to the brain of some cancer types is an intriguing phenomenon whose biological mechanisms remain to be clarified. The so-called ‘seed and soil’ hypothesis could be defined as the biological mechanisms that permit the development of the diffusion of cancer in the central nervous system (CNS). Signalling through HER2, EGFR, PI3K, mTHOR, HPSE and Notch1-related pathways might mediate specific biological processes important to tumour growth and metastatic spread, including angiogenesis, epithelial-mesenchymal transition and resistance to standard therapeutic treatments. Other than the use of antiangiogenic agents, the exploitation of these biological processes for therapeutic intervention in the context of BM remains mostly limited to preclinical studies (3,4).
The solid tumors most frequently associated with BM are the lung, breast, melanoma and kidney. The treatment approaches for BM include surgical resection and radiation therapy, including stereotactic radiosurgery (SRS); to date, no standard cytotoxic chemotherapy exists for the treatment of CNS metastases. Instead, the patients in whom the disease is not amenable to local control with surgery or radiation are typically treated using the same cytotoxic chemotherapy employed for the treatment of extracranial disease. Cytotoxic agents with good CNS penetration, such as carboplatin, cisplatin, topotecan, irinotecan, procarbazine, temozolomide are also employed for empiric therapy, even in cases in which these agents are not the standard therapy for the primary tumour site. The improvement of the knowledgement of tumour biology has led to the identification of specific molecular drivers of cancer development and progression. Of note, insertions and/or deletions within the EGFR gene and EML4-ALK chromosomal translocation in lung cancer, BRAF mutation in melanoma and overexpression or genic amplification of HER2 protein in breast cancer distinguish different and distinct subsets of cancer amenable to specific and unique treatment approaches (5,6). In particular HER2 overexpressing breast cancer predicts the extremely variable prognosis of BC patients who develop CNS metastases. In fact, a median survival greater than 1 year from the time of development of BMs has been consistently reported for HER2-positive (HER2+) BC patients (7), likely reflecting the great efficacy of HER2- directed therapies administered after CNS progression for the treatment of systemic disease, principally based on the use of the monoclonal antibody trastuzumab in association with chemo- or endocrine therapy (7,8). Conversely, the survival of patients with CNS metastases from HER2- negative BC is usually poorer (9), with the worse outcome observed in patients with BMs from HER2-negative/hormone receptor negative (triple negative) BC, where not only median survival from the time of diagnosis of BMs has been reported to be less than 6 months (9,10) but also the delivery of systemic chemotherapy after the development of CNS metastases has been shown to have little or no impact on overall prognosis (8). These considerations are crucial in order to set the stage for discussing novel systemically delivered targeted therapies that are under development for the treatment of BMs from breast cancer [for example the new antiHER2 molecules: pertuzumab and trastuzumab/emtansina (T-DM1)].
Molecular characterization and targeted therapy for these solid tumors malignancies have important implications for BM. First of all that specific molecular drivers lead to increased predisposition of cancer cells to invade the CNS is plausible. Furthermore, in pre-clinical models, deregulated EGFR and HER2 signalling in co-operation with activated HGFR (also known as c-Met) pathway induce an epithelial-to-mesenchymal phenotype transition, which can promote increased metastatic potential and higher likelihood of brain involvement (11).
Secondly, the use of targeted agents is generally associated with superior efficacy and survival improvement, which has led to the approval of these agents for specific tumour subtypes. Consequently, increased longevity of patients with cancer treated with targeted biological agents might also increase the likelihood of BM over the course of the disease. This eventuality might result from failure of the targeted agents to eradicate micrometastatic deposits in the brain due to limited penetration through the blood-brain-barrier (BBB), or because of selective pressure leading to the emergence of treatment-resistant clones with increased capacity for invasion and metastasis to distant sites. Paradoxically, the limited penetration of some targeted therapies into the brain could result in intracranial metastatic deposits that remain sensitive to these agents, even in the context of the development of drug resistance within the extracranial tumour compartments. Conversely, exposure of intracranial tumour deposits to subtherapeutic drug concentrations might promote the early development of drug resistance and isolated disease progression in the brain, while the extra-cranial disease remains sensitive to treatment.
Finally, and most relevant is the possibility of incorporating biologically targeted therapy into the management paradigm for BM in patients whose tumours harbour genetic alterations that render them sensitive to such agents.
In this review we analyze clinical measurement to better define outcome of drugs for BM from solid tumors in the new era of biological and immunological therapies.
RANO criteria: between the drug and the patient
In most of the time patients with BM are still excluded from clinical trials. There remains today a critical and delicate point the inclusion of patients with BM in the clinical trials, although the advent of new target molecules and immunotherapy in some types of solid tumors is demonstrating the effectiveness of the treatments the outcome of disease.
The primary end point of phase 3 trials is to show a higher benefit than standard of care, either through improved survival or quality of life. The overall survival has usually been the primary endpoint of phase 3 trials of BM (12-14). Development of treatments that prolong overall survival by a clinically meaningful extent is a worthy goal, shared by both patients and health-care providers. However, patients with BM often have coexisting extracranial disease that can have a major effect on survival, independent of CNS disease control.
The progression free survival (PFS) is often used as the primary endpoint in phase 3 trials examining solid tumour metastases outside of the CNS. However, there are several reasons why intracranial PFS might not be a sufficient endpoint in BM treatment trials. In studies of SRS with or without whole-brain radiotherapy, PFS was considered a not so adequate end point able to capture the risk-benefit profile of the two treatment because of concerns about neurocognitive decline associated with whole brain radiotherapy (WBRT). Furthermore the progression can also be difficult to define after SRS because radiographical findings might show effects of radiation and radionecrosis. PFS can be affected by the ability of the studied treatment to affect extracranial progression. In the case of radiotherapy trials, concurrent or sequencing systemic therapies might confound interpretation of results (15).
On the other hand the primary aim of phase 2 trials is to define the activity of a treatment that would be able to move to a definitive phase 3 trial. In the phase 2 study the endpoints that have been used are objective response rate, the PFS, the time to failure, quality of life and neurocognitive outcomes and quality of life. All of these endpoints, when applied to patients with BM, have several strengths, limitations, and unresolved issues.
PFS
In patients with known BM or with BM discovered during the study, assessment of PFS can be more challenging. Considerations are whether and how to define and incorporate progression in CNS versus non-CNS sites in the trial design, endpoints, and analysis.
The first consideration is the actual assessment of intracranial progression. An important complicate factor for patients with BM given SRS is how to distinguish between tumour progression and radiation necrosis (RN). A second consideration is whether to treat intracranial and extracranial disease separately or together, and how this strategy should vary on the basis of techniques used for the BM treatment and whether the main focus of the trial is BM, extracranial benefit, or both. The typical patient with known BM could have both CNS and non-CNS target lesions and is at risk of progressing in either, or both, sites.
Therapies directed only towards the CNS would not be expected to affect extracranial disease. So, in case of locoregional treatment (e.g., surgery, SRS, with or without WBRT), consideration of intracranial PFS as a key endpoint is reasonable. Patients in phase 3 studies should be stratified according to extracranial disease status, and statistical approaches that use competing risks should be considered to account for patients who die of systemic disease before CNS progression. Overall PFS including both intracranial and extracranial progression events should also be reported as a secondary endpoint. If intracranial PFS is chosen as an endpoint, descriptions of concurrent and sequential systemic treatment is recommended because of the potential for a change in systemic treatment to affect CNS disease.
Because intracranial PFS might be questioned as primary endpoint without evidence of a corrispective improvement or mantenance of neurological symptoms, quality of life, or overall survival, secondary endpoints assessing these additional factors could provide valuable information.
For trials with chemotherapy or biological drugs, overall PFS is usually an end point more appropriate than intracranial PFS.
Response rate
To date, response criteria have been inconsistent in trials of patients affected by BM from solid cancers. Trials have used either standard criteria developed for solid tumours [e.g., Response Evaluation Criteria in Solid Tumors (RECIST)] (16-18) or those disegned for high-grade gliomas (19,20), often with additional modifications. As well known, World Health Organization (WHO) criteria, based on bidimensional measurements, were used to assess response to treatment (18). Because a 30% decrease in unidimensional measurement and a 50% reduction in bidimensional product correlated closely in a test-set population (=0.95 for overall response), RECIST used unidimensional measurements as the standard (16,21). Patients with either primary brain tumours or BM from solid tumours were not well represented in the development of RECIST. Neither steroid use nor neurological symptoms are included in the evaluation of outcome disease. So, RECIST criteria have been used to measure overall disease burden, and the individual sites of disease have not been analysed separately. Thus, for a patient with both brain and liver metastases, target lesions could include representative lesions from both organs, and response would need the sum of all target lesions to decrease by at least 30%.
In order to address some of the limitations of WHO criteria, Macdonald and colleagues (19) proposed a standard response assessment in malignant glioma. Their criteria have been widely used in neuro-oncology trials, and in some trials of patients with BM (19). Several of the innovations of the Macdonald criteria include the specification of so-called enhancing tumour (e.g., tumour that enhances with gadolinium contrast), as suitable for measurements, and the inclusion of steroid use and neurological symptoms in the definition of response (19). However, these criteria do not address issues of response and progression in systemic sites of disease.
The RANO group (22) published a critical assessment of the Macdonald criteria and defined clinical situations in which clarification and updates were needed. Furthermore, RANO published an updated response assessment criterion for malignant gliomas (MGs) (20). For partial response, at least a 50% decrease in the sum of products of perpendicular diameters of all measurable enhancing target lesions sustained for at least 4 weeks is needed. Furthermore RANO criteria require lesions that are stable or improving when imaged with non-enhancing T2-weighted or fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), and no increase in steroid dose from baseline scan and stable or improved clinical status. However, because the RANO guidelines were designed and developed for evaluation of treatment in malignant gliomas, some issues for patients with BM have not been addressed.
Because none of these criteria were specifically designed to assess BM, the limits of the modern era concerning the evaluation of drug response for BM is the heterogenous use of existing criteria in different ways.
The more frequent areas of difference include the type of measurement (unidimensional, bidimensional, or volumetric), degree of tumour change needed for response and progression, requirement for confirmatory scans, inclusion of corticosteroid use and neurological symptoms, and consideration of extracranial disease status in response definitions.
Imaging evaluation
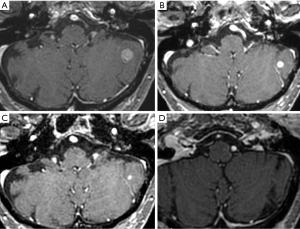
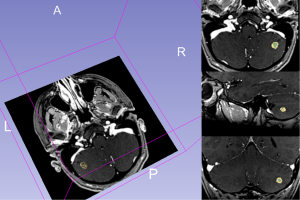
In the therapeutic management of BM imaging provides at the identification, diagnosis, size, localization and evaluation of the treatment response. MRI exhibits superior sensitivity to computed tomography (CT) for small lesions identification and to evaluate their precise anatomical location; for this reasonance MRI represents the first choice in the evaluation of BM (23). The CT scan will be made only in case of MRI’s contraindication. Beyond diagnosis of BMs, CT, MRI and PET-CT, may be used to monitor response to treatment as part of clinical and radiological follow up; this may be immediate, as in post-surgery, to determine if there has been a complete resection or residual, or later in the evaluation of the response to chemo or radiotherapy. In the evaluation of response to treatment, MRI shows superior accuracy in comparison to CT; PET-CT is useful in particularly in cases of BMs underwent to radiotherapy, in the differential diagnosis between recurrence or radionecrosis (23). In the imaging evaluation of the response to metastatic brain treatment, quantitative measurements are commonly limited to define of lesion size with RECIST based on one-dimensional measure (LD only) (Figure 1) or WHO that is based on two-dimensional long-axis measurements (product of LD and greatest perpendicular diameter). Another method may be to measure lesion volume and the variation after treatment is the volumetric measurement; in this case, using specialized volumetric software, is possible to evaluate whole volume of the metastases, on imaging, generally in the images obtained after infusion of contrast medium, which permits to separate the lesion from vasogenic oedema. However, this method is time-consuming and the software is not available to most radiologists; for this resonance reporting of lesion volume is not standardized. Figure 2 shows the volumetric method for evaluating lesion size.
Response evaluation: functional and metabolic imaging
SRS is a therapy of choice of single or maximum three metastases; during stereotactic radiation therapy, very high doses of radiation are delivered to the target, with rapid falloff over a few millimeters. In the cases of BM underwent to SRS the enlargement of an enhancing lesion may be due to tumor progression or RN (24). RN of the brain is a syndrome of brain coagulative and fibrinoid necrosis and cortical irritation that occurs following radiotherapy with peaks in a delayed fashion at 9–12 months post-procedure. Vasogenic cerebral edema secondary to necrosis occurs and can affect surrounding brain function. The differential diagnosis between radionecrosis/recurrence represents for radiologist, a great problem, because on the morphological CT or MR, recurrence and RN show the similar aspect, generally as a ring enhancement after contrast medium injection, associated with edema and/or mass effects (24-26). In the cases of differential diagnosis is necessary to use the functional imaging techniques, such as CT-perfusion, dynamic susceptibility contrast (DSC) MR imaging, dynamic contrast-enhanced (DCE) MR imaging (Figure 3), diffusion-weighted MR imaging (DWI), MR-Spectroscopy or metabolic techniques as PET-CT with 11C-methionine or 18F-fluorodeoxyglucose; although these imaging techniques can provide qualitative and quantitative imaging parameters that allow pathophysiologic correlation; consensus does not exist (27-30). In this regard, several studies have highlighted as perfusion parameters (elaborated either with MR or CT) may help in differentiating tumors recurrence from RN, since recurrence generally shows an increased cerebral blood volume (CBV) while this parameter is relatively low in the radionecrosis (26-28). The two major techniques, on perfusion imaging, currently used are DSC MR (T2*-weighted first-pass, dynamic susceptibility contrast-enhanced perfusion) and DCE MR (T1-weighted steady-state dynamic contrast-enhanced). In DSC MR imaging, the signal measured is due to the susceptibility T2 or T2* effect induced by the injected contrast agent; is possible to obtain some information about relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF) method. The DCE-MRI procedure typically consists of intravenous injection of contrast agent followed by the repeated acquisition of T1-weighted images, providing measurements of signal enhancement as a function of time. The enhancement kinetics can be used to extract semi-quantitative information, about blood volume and blood flow (nIAUGC) or quantitative parameters as permeability (Ktrans), extracellular-extravascular space (Ve). In the evaluation of the response to treatment after SRS in the BMs, DSC-MRI is the method of choice for perfusion imaging (31). However, it has been shown that DCE-MRI can combine perfusion and permeability measurements by using a sufficiently long acquisition time, to capture slow interstitial uptake with high temporal resolution early to capture the first pass of the contrast bolus. CT-perfusion technique generate quantitative parameters such as relative blood volume, relative blood flow and mean transit time and represent a valid alternative to MR perfusion. In the clinical setting the common analysis used in the evaluation of functional imaging, on CT and MRI, is the region-of-interest (ROI). Tumor tissue is identified on morphological images (on MRI on contrast enhanced T1-weighted or T2-weighted images) and simultaneously on the perfusion images; usually, are placed on a single axial section (the more representative of the disease) in tumor region of maximum rCBF (or rCBV) levels; mirrored regions in the contralateral hemisphere are automatically drawn, after having defined the median line; the CBV values inside the ROI are then divided by the average CBV inside the contralateral region, to obtain the distribution of the normalized CBV values: nCBV (normalized CBV). Another system of perfusion metrics, as hysterogram or parametric response map or clustering are currently undertaken in the research settings. In study of Vidiri et al. (29), were compared different perfusion metrics obtained from the analysis of the CBV map with CT-perfusion, in order to investigate the possibility of improving the diagnostic accuracy of this technique in differentiating BM recurrence from RN. The metrics based on the histograms were found to have higher predictive value. DWI represents another technique that can be used in the differential diagnosis between recurrence/radionecrosis. DWI may reflect the tissue cellularity and it can be easily incorporated into routine MRI protocols. DWI is based on differences in water mobility in different tissues, which can be quantified into an apparent diffusion coefficient (ADC). ADC readings from metastases treated with radiosurgery, taken immediately post treatment, may be tracked to determine if the lesion is responding to therapy, showed as increasing ADC as compared to a recurring or necrotic lesion; the initial ADC value may also predict final response to the treatment because in the good responder patients, the ADC value, is generally low in the baseline (24). PET using 2-deoxy-[F]fluoro-D-glucose (FDG) has been considered for the evaluation of BM but this tracer has been used with variable success because of the high normal glucose metabolic activity of the brain. For example, in a study of 48 patients with lung cancer and BM, 33% of the brain lesions could not be clearly detected by FDG PET, although all primary lung lesions were hypermetabolic; furthermore, another limitation is a small lesion size. PET using 11C-methionine may be effective in differentiating recurrent metastatic brain tumor from radiation-induced changes with a sensitivity of 78% and a specificity of 100% in one study respectively, and in a second, larger study, a sensitivity of 79% and a specificity of 75% (32). Another tracer, that can be use is the O-(2-[18]F-fluoroethyl)-L-tyrosine ([18]F-FET), differentiating local recurrent BM from RN; recently for this tracer has been reported a sensitivity of 74% and a specificity of 90% (33). Another tracer used in the patients with BMs, in particularly in the differential diagnosis between radionecrosis/recurrence after SRT, is the [18]F-fluorodopa ([18]F-FDOPA) PET. Lizarraga et al. (34) have been demonstrated that FDOPA PET could distinguish radionecrosis from recurrence with a high diagnostic accuracy 83.1% (sensitivity, 81.3%; specificity, 84.3%) in a group of the patients population in whom the recurrence was suggested by MR and the evaluation FDOPA PET was highly prognostic of PFS. In the study of Cicone et al. (35), the F-DOPA PET was compared with perfusion-MRI obtained with DSC technique; in this study, with a cut-off value of 1.59, a sensitivity of 90% and a specificity of 92.3% were achieved, in differentiating radionecrosis from recurrence, with an accuracy of 91.3%. rCBV derived from perfusion-MR was available for comparison in 37 of the 46 metastases. Overall accuracy of rCBV was lower than that of all semiquantitative PET parameters under study. The best differentiating rCBV cut-off value was 2.14; this yielded a sensitivity of 86.7% and a specificity of 68.2% (accuracy 75.6%).
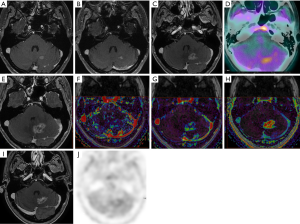
There is strong interest in validating biomarkers derived from advanced non-invasive imaging techniques that could be predictive of the treatment efficacy and overall survival. DCE MRI and Perfusion-CT appear to be appropriate and powerful techniques to measure changes in the underlying tumor vasculature resulting from antiangiogenic therapy. In particular, DCE-MRI has the advantage of quantifying permeability parameters, such as the transfer constant Ktrans which may help in clarifying the mechanism of action of bevacizumab, an antiangiogenic monoclonal antibody active against lung, brain, ovary colon-rectum and ovary cancer. In our studies of MGs recurrence patients (36) treated with bevacizumab and in which DCE MRI and Perfusion CT was used for assessment, we have shown that responder patients at the 3-month RANO assessment showed a significantly lower median nIAUGC on DCE MRI and nCBV on CT Perfusion at baseline compared with nonresponder patients. On CT Perfusion for an initial mean nCBV greater than 2.5, the normalization effect of antiangiogenetic drugs on the tumor vascularity, seems to be less efficient, suggesting that a high perfusion at baseline may correspond to reduced activity of the anti-angiogenic agent. After one single dose of antiangiogenetic drugs the responder patients showed a reduction of nIAUGC on DCE MRI and rCBV on CT Perfusion compared with non responder patients. Quantification of specific tumor subvolumes with increased values of nIAUGC and Ktrans, using the histogram approach, showed the potential for improving the diagnostic accuracy of DCE-MRI in differentiating responder and nonresponder patients. These concepts could be translated on the evaluation of the response to antiangiogenetic drugs in BMs.
Immunotherapy and imaging evaluation: route change
Another important chapter covers the response to immunotherapy treatments, where the immune-related response criteria (irRC) are considered as the gold standard for evaluating the clinical response of immunologic agents (37). The irRC utilizes bidimensional measurements, quantifying the tumor dimension using a product of the longest diameter and the longest perpendicular diameter. In the irRC the complete response is the disappearance of all lesions in two consecutive observations not less than four weeks apart; the partial response is a ≥50% decrease in tumour burden compared with baseline in two observations at least four weeks apart; the stable disease is a 50% decrease in tumour burden compared with baseline cannot be established nor 25% increase compared with nadir; the progressive disease is at least 25% increase in tumour burden compared with nadir (at any single time point) in two consecutive observations at least four weeks apart. In the recent article by Hodi et al. (37), the authors evaluated immune-related responses in patients treated with PD-1 inhibitor therapy and reported the overall survival data in correlation with the irRC and RECIST assessments. Their results indicated that the conventional RECIST assessment alone may underestimate the benefit of the treatment. In this study, furthermore, the authors has been reported two types of pseudoprogression: (I) early pseudoprogression with ≥25% increase at 12 weeks that is not confirmed as PD at the next assessment; and (II) delayed pseudoprogression with ≥25% increase after 12 weeks that was not confirmed as PD at the next assessment. The definition of pseudoprogression is not yet clear and the study defined subsequent tumor reduction as “not confirmed as PD at the next assessment”. However using the irRC criteria the measurement variability represent the important issue; for this resonance prior study have been demonstrated that the unidimensional irRC provides highly concordant assessment compared to bidimensional irRC with less measurement variability.
Conclusions
The difficulty of treatment of BM is associated to the current trouble of measuring the effectiveness of care especially with the advent of biological and immunological drugs.
There are still some points to be analyzed. First the uncommon inclusion of BM in clinical trials especially phase 3 studies, which makes arduous the use of some new drugs in clinical practice. Second, in the current practice it remains a limit the uncommon use of criteria evaluation of treatment response criteria (RANO for chemotherapy and biological molecule and irRC for immunological treatments) Among the imaging techniques used for efficacy evaluation (CT, MRI and PET-CT), MRI remains superior for accuracy while PET-CT is useful in that cases in which BM are submitted to locoregional treatments in order to differentiate recurrence or radionecrosis. Newer functional imaging such as CT-perfusion, DSC MR imaging, DCE MR imaging, diffusion-weighted MR imaging and MR-spectroscopy can provide qualitative and quantitative imaging parameters that allow pathophysiologic correlation. In the evaluation of the response to immunotherapy treatments, the irRC are considered as the gold standard.
This suggests the importance of subjecting the patient to a screening with BM using the most modern possible tools so to allow for a more effective therapeutic evaluation especially through the use of new biological and immunotherapeutic molecules.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hall WA, Djalilian HR, Nussbaum ES, et al. Long-term survival with metastatic cancer to the brain. Med Oncol 2000;17:279-86. [Crossref] [PubMed]
- Maclean J, Fersht N, Singhera M, et al. Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat Oncol 2013;8:156. [Crossref] [PubMed]
- Gril B, Palmieri D, Qian Y, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res 2011;17:142-53. [Crossref] [PubMed]
- Palmieri D, Fitzgerald D, Shreeve SM, et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res 2009;7:1438-45. [Crossref] [PubMed]
- Shaw AT, Mehra R, Kim DW, et al. Clinical activity of the ALK inhibitor LDK378 in advanced, ALK-positive NSCLC. J Clin Oncol 2013;31:abstr 8010.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Metro G, Fabi A. New target therapies for brain metastases from breast cancer. Curr Cancer Drug Targets 2012;12:210-7. [Crossref] [PubMed]
- Niwińska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer 2010;116:4238-47. [Crossref] [PubMed]
- Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 2008;112:2359-67. [Crossref] [PubMed]
- Anders CK, Deal AM, Miller CR, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer 2011;117:1602-11. [Crossref] [PubMed]
- Nie F, Yang J, Wen S, et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer 2012;118:5198-209. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys 1997;39:571-4. [Crossref] [PubMed]
- Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol 2006;24:106-14. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207-14. [Crossref] [PubMed]
- Macdonald DR, Cascino TL, Schold SC Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277-80. [PubMed]
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963-72. [Crossref] [PubMed]
- James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst 1999;91:523-8. [Crossref] [PubMed]
- Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 2015;16:e270-8. [Crossref] [PubMed]
- Zakaria R, Das K, Bhojak M, et al. The role of magnetic resonance imaging in the management of brain metastases: diagnosis to prognosis. Cancer Imaging 2014;14:8. [PubMed]
- Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics 2012;32:1343-59. [Crossref] [PubMed]
- Narang J, Jain R, Arbab AS, et al. Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro Oncol 2011;13:1037-46. [Crossref] [PubMed]
- Hoefnagels FW, Lagerwaard FJ, Sanchez E, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 2009;256:878-87. [Crossref] [PubMed]
- Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 2010;99:81-8. [Crossref] [PubMed]
- Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008;49:694-9. [Crossref] [PubMed]
- Vidiri A, Guerrisi A, Pinzi V, et al. Perfusion Computed Tomography (PCT) adopting different perfusion metrics: recurrence of brain metastasis or radiation necrosis? Eur J Radiol 2012;81:1246-52. [Crossref] [PubMed]
- Chernov M, Hayashi M, Izawa M, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg 2005;48:228-34. [Crossref] [PubMed]
- Sourbron SP, Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR Biomed 2013;26:1004-27. [Crossref] [PubMed]
- Tsuyuguchi N, Sunada I, Iwai Y, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg 2003;98:1056-64. [Crossref] [PubMed]
- Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 2012;53:1367-74. [Crossref] [PubMed]
- Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 2014;55:30-6. [Crossref] [PubMed]
- Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging 2015;42:103-11. [Crossref] [PubMed]
- Piludu F, Marzi S, Pace A, et al. Early biomarkers from dynamic contrast-enhanced magnetic resonance imaging to predict the response to antiangiogenic therapy in high-grade gliomas. Neuroradiology 2015;57:1269-80. [Crossref] [PubMed]
- Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol 2016;34:1510-7. [Crossref] [PubMed]


