Non-neuronal cholinergic system in airways and lung cancer susceptibility
Introduction
Acetylcholine (ACh) is one of the main regulators of airway function and one of the most powerful known bronchoconstrictors and stimulators of secretion. However it is also involved in regulating less acute mechanisms, such as airways remodeling which takes place in pathologic settings and in response to immunomodulation (1). As a consequence, pharmacological manipulation of cholinergic signaling—mostly inhibitory—is a key option among treatments of common lung diseases, such as chronic obstructive pulmonary disease (COPD) and asthma. On the other hand, it is now well documented that various non-neuronal cells are capable of ACh synthesis and release (e.g., keratinocytes, lymphocytes, placental trophoblast and endothelial cells) and that non-neuronal ACh is also present in the airway epithelium where it is believed to regulate cell proliferation (2) (Table 1).
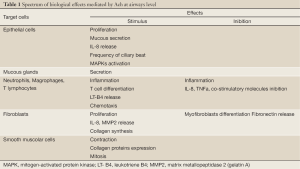
Full Table
On the airway surface, at least twelve types of epithelial cells can be identified whereas other five types can be found in the airway glands; among them differentiating or intermediate elements can be identified too. The most important function of the respiratory epithelium is mediated by the ciliated cells which provide the driving force for mucociliary clearance, namely the cleaning of the airway surface from inhaled particles by transporting a mucous layer towards the larynx. Ciliated cells constitute form 32% to 55% of tracheal epithelial cells (3). They feature columnary structure in the trachea and large bronchi (approximately 20 µm long and 7 µm wide) whereas decrease in height towards small bronchi and bronchioli. They also structure microvilli that protrude into the lumen. Notably on their surface compartment is located the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which—when structurally altered in consequence to point mutations affecting the corresponding coding gene—is causally linked to cystic fibrosis onset. Submucosal glands producing the carbohydrate-rich glycoproteins (‘mucins’) and lipids of the mucous layer are known as ‘non-ciliated cells’. Classical goblet cells account for 9% in the human trachea and are almost absent from human distal bronchioli (3). Smaller airways contain cells with protruding apical region harboring few microvilli, abundant smooth or rough endoplasmic reticulum, and secretory granules smaller than those found in goblet cells: in distal airways they identified the Clara cells. Basal cells account approximately for 30% of tracheal epithelial cells and are found in larger airways only. Finally, there are—rather infrequent—specialized epithelial cells that are capable of producing conspicuous amounts of acetylcholine; among these there are neuroendocrine cells that origin from different precursors from those of other epithelial cells. They generally occur solitarily in the airway epithelium, or clustered as neuroepithelial bodies (NEBs) preferentially located at bronchial branching (4). They contain, in their basal compartment, dense core granules, mainly composed by bioactive amines and neuropeptides. NEBs prevail in embryo and during the neonatal period and, by functioning as oxygen sensors, are correlated with lung maturation (5). The oxygen sensor function, however, has not been completely established yet; however more recent evidence suggests that NEBs are myelinated vagal afferents belonging to the subpopulation of the myelinated mechano-sensitive vagal airway receptors (6). The role of solitary pulmonary neuroendocrine cells is still partially unclear, probably they are involved in fetal and newborn lung development including regulation of branching morphogenesis, cellular growth and maturation. It has been demonstrated that, in adult mice, they are associated with stem cell niches in proximal and distal airways, and it has been proposed that they contribute to the protection of stem cells from environmental agents thus promoting stem cell renewal (7). Finally, an equally infrequent cell type is represented by the so-called ‘brush cells’ (characterized by an apical brush of microvilli) (8). Tracheobronchial brush cells express components of the taste-signaling cascade and are hence considered chemosensory cells. It has also been proposed that they may sense bacterial colonization and are useful to initiate defense mechanisms (9).
ACh synthesis and recycling
Cholinergic nerve fibers
Acetylcholine is synthesized in the axoplasm by choline acetyltransferase (ChAT) from choline—taken up from the extracellular space—and acetyl coenzyme A (acetyl-CoA) which is produced in mitochondria. This uptake represents the rate-limiting step in neuronal ACh synthesis and it is realized through a high-affinity choline transporter (CHT1) (10). Once generated in the axoplasm, the ACh is stocked into small synaptic vesicles. The process is mediated by the vesicular acetylcholine transporter (VAChT), a twelve transmembrane domain protein which acts as a H+/ACh exchanger. Inside each vesicle there are up to 10,000 molecules of ACh bound in a matrix enriched with proteoglycan SV2. The depolarization of the nerve terminal triggers exocytotic release of ACh from the vesicles into the extracellular space; once there, ACh is able to interact with two classes of cholinergic receptors: (I) metabotropic muscarinic receptors (MR): G protein-coupled receptors with seven transmembrane domains, having five different isoforms (M1-M5); (II) ionotropic nicotinic acetylcholine receptors (nAChR) which are cationic channels having two ACh binding sites and structured as hetero- or homopentamers (11,12). The action of ACh terminates quite rapidly being spatially limited by cleavage into acetate and choline via the acetylcholine-esterase (AChE). This efficient enzyme is synthesized by cholinergic neurons themselves and guarantees equilibrium between ACh production and its degrading capacity. Choline is thus taken up again at the nerve terminal via CHT1, and a new cycle of ACh synthesis and release is to begin (1).
Non-neuronal cells
Non-neuronal ACh synthesis system identifies a philogenetically old process which is detectable also in bacteria and plants (1,13). Indeed some of the enzymes and transporters of cholinergic neurons have evolved relatively recently and cannot be found in the non-neuronal cholinergic system. Each cell includes an uptake mechanism for choline which represents a necessary element for the synthesis of plasma membrane lipids, in particular phosphatidylcholine. There are a number of plasma membrane choline transporters, but only few cholinergic non-neuronal cells are capable of expressing the high-affinity choline transporter CHT1. An alternative way for ACh synthesis is provided by carnitine acetyltransferase (CarAT) which, although in principle less efficient than ChAT, plays a key role in ACh synthesis in skeletal muscle fibres (14). VAChT and vesicular storage mechanisms for ACh, in non-neuronal cholinergic cells, are still unclear, but it is widely accepted that they do not imply exocytosis. In fact, there is evidence of ACh release via plasma membrane-bound polyspecific organic cation transporters (OCTs) (15). These electrogenic transporters are bidirectional and their driving forces are represented by substrate concentration and membrane potential. A proteolipid known as ‘mediatophore’ is also involved in the release of ACh, either directly from the cytoplasm or by forming the fusion pore between the synaptic vesicle and the plasma membrane. It is part of the vacuolar H+-ATPase (V-ATPase, V0 subunit c) that is predominantly targeted to acidic organelles such as lysosomes, endosomes and secretory vesicles (16,17). V-ATPase complex is localized to the plasma membrane in human lung microvascular endothelial cells, so that ‘mediatophore’ could mediate ACh release from these cells (18). Once released, ACh can be cleaved by esterases which are less specific than AChE; among them the most important is butyrylcholinesterase (BChE) (19).
Choline transporters in the airway epithelium
It has been demonstrated that several systems of choline uptake and transport can be detected, featuring a cell-specific distribution. For instance, the high-affinity choline transporter CHT1, known from the nervous system, is localized to the apical membrane of the ciliated cells in the rat trachea (20). This finding has been validated by in situ-hybridization, Western blotting of abraded tracheal epithelium, and RT-PCR of tracheal epithelium obtained by laser-assisted microdissection which have led to the identification of a molecule featuring the same biochemical properties and immunophenotype of CHT1. Overall these data put in evidence the existence of high affinity uptake of choline from the airway lining fluid into ciliated cells via a transport system that has been originally thought to be specific for neurons; nevertheless it remains still unclear how other epithelial cholinergic cells, lacking CHT1, are capable of ACh synthesis. In fact, airway epithelial cells express additional choline transport systems, which can work alone or in parallel with CHT1 in specific cell types. For example, A549 cells (human lung adenocarcinoma cells) co-express, in addition to CHT1, a sodium-independent choline transport system, that relies on a transmembrane H+ gradient and which is sensitive to amiloride (21).
Apart from CHT1, choline transporters can be classified into two large families: choline-specific transporter-like proteins (CTL family) and polyspecific organic cation transporters (OCT family); members of both families are expressed in the lung. CTL1, the most relevant member of the CTL family, detectable by Western blotting in human lung extracts, is expressed in A549 cells and participates predominantly to choline uptake in this cell line (22). Among the polyspecific OCT family members, OCT1 and OCT2 (but not OCT3) do transport choline: OCT1 is expressed in the mouse, rat and human bronchial epithelium, and immunohistochemical stain has showed a predominant localization in the apical membrane of ciliated cells (23). OCT1 expression, in the airway epithelium, is cell type-specific: rat studies confirmed that OCT1-immunoreactivity is selective for ciliated cells but absent in secretory, brush and basal cells (23).
On the other side, OCT2 is expressed in human, but not in mouse, bronchial epithelium. In human bronchi, OCT2-immunoreactivity is predominant in the luminal membrane of ciliated cells, rarely found in basal cell membranes, and absent from goblet cells (23). In OCT1/OCT2 double-knockout mice, tracheal epithelial ACh content is quite elevated instead of being, as expected, reduced; thus, despite OCT1 and OCT2 are capable of choline translocation across the plasma membrane, they are not crucial for providing choline for epithelial ACh synthesis (23). In conclusion, there is a multiplicity of choline uptake systems in airway epithelial cells with a cell type-specific distribution and a distinct apical vs. basolateral polarization.
ACh synthesis in the airway epithelium
Notwithstanding the presence of choline acetyltransferase enzyme (ChAT—responsible for the production of Ach—has been undoubtedly documented), the real identity of the ACh synthesizing enzyme in the individual airway epithelial cell types is nowadays not completely known. First, it is important to underline that there is a great diversity among ChAT variants, although they are all encoded by the same gene. These differences are so marked that the various ChAT variants react with different antisera. The mammalian ChAT gene contains three non-coding exons (termed R-, M- and N-exon in mouse and rat models) and, depending on species, 15-16 coding exons. The sequence encoding for VAChT is inserted between the two not transduced exons R- and N-. This peculiar gene structure codifying for ChAT and VAChT is known as ‘cholinergic gene locus’ (24). Multiple transcripts derive from alternative splicing processes; a numbers of these variants can be found in mouse and rat models, and at least six are known in humans. In the central nervous system, all of these variants are expressed with the M-type ChAT-mRNA usually dominating, while in the bronchial epithelium, expression of non-coding exons M- (in rat), N- and S- (in monkey) has been identified. Different ChAT protein variants can also result from alternative splicing in the coding region. For example, in central nervous system, in addition to the 69 kDa (cChAT), a protein deriving from the removal of 6-9 coding exons can be identified: this form is prevalent in peripheral autonomic neurons (pChAT); whereas, in the rat tracheal epithelium, only the complete form of the enzyme has been detected. These findings have been confirmed by immunohistochemistry studies, that have documented the presence of cChAT in all epithelial cell types of trachea; in more distal airways, cChAT-immunolabelling of ciliated and secretory cells was generally less intense than in the trachea, whereas endocrine cells and brush cells were particularly cChAT-immunolabelled (25,26). Within tracheal ciliated cells, a more intense labeling of the apical cytoplasmatic region was registered: this evidence suggest an earlier ACh synthesis in trachea then in distal bronchi. In these cells cChAT is located close to the high-affinity uptake system for choline CHT1. The latter allows the concentration of the entire ACh synthesizing machinery at the apical side of the ciliated cell thus suggesting its luminal release. These data altogether point to a rather homogenous expression of a single variant of ChAT (ChAT translated from M-type ChAT m-RNA) in various airway epithelial cell types. There are, however, several data on protein level that indicate a more complicated situation. In particular, in bronchial epithelial extracts, ChAT-labeling of human bronchial epithelium with an antibody that recognized 54 and 41 kDa proteins has been reported (27). These controversial findings cannot be entirely justified through a possible cross reaction with a closely related or even unrelated protein, but, in this regard, several detailed studies are still in progress.
Mechanisms of ACh release in the airway epithelium
In neurons, VAChT shuffles ACh from the axoplasm into synaptic vesicles. The particular ‘cholinergic gene locus’ plays a key role in orchestrating the coordinate expression of ChAT and VAChT thus balancing production and release of ACh. In the airway epithelium, VAChT labeling has been demonstrated by immunohistochemistry in trachea and bronchial neuroepithelial and secretory cells, as well. Human small cell lung carcinoma cell lines, derived from airway neuroendocrine cells, express VAChT along with ChAT, and ACh release from these cells is sensitive to vesamicol (a VAChT inhibitor) (28). Ciliated cells, however, seem to utilize a non-vesicular ACh release mechanism. OCT1 and OCT2 are localized at the apical membrane of ciliated airway epithelial cells while OCT3 is concentrated at the basolateral membrane of several cell types. This distribution permit to hypothesize that direction (release or uptake) of ACh is determined by concentration gradient and membrane potential. The apical localization of OCT1 and OCT2 in airway ciliated cell suggests a complete cycle of ACh synthesis, release and reuptake of choline between the ciliated cell and the luminal airway lining fluid.
On the other hand, the role of OCT3 is less clear: probably, OCT3 requires expression of additional proteins to serve as an ACh transporter (23). These polyspecific transporters are the target of a number of drugs which either compete with transport of other cations or block transport without being transported themselves. Very important for airway pharmacology, nicotine and corticosteroids (corticosterone, fluticasone, and budesonide) could block ACh release by OCT1 and OCT2 in vitro. Consequently, inhibition of non-neuronal ACh release is a non-genomic effect of corticosteroids that clearly discriminates non-neuronal from neuronal cholinergic mechanisms in the airways (29). Within respect to ‘mediatophore’, its occurrence and distribution in the airway epithelium has not been deeply investigated yet. Altogether, the currently available data suggest that ACh release in the respiratory epithelium can occur through: (I) vesicular basal release by neuroendocrine and possibly brush cells; (II) vesicular luminal release by secretory cells or (III) apical concentration- and membrane potential-driven transmembrane release from the cytoplasm of ciliated cells.
Mechanisms of ACh degradation in the airway epithelium
As discussed above, in the nervous system the cholinergic signaling finishes at short distances from the site of ACh release since ACh is divided into acetate and choline through the action of the AChE. A number of other esterases, among which BChE, also coexist. The high speed of AChE effect allows some considerations. First it should be noted that the amount of the ACh generated in the airways is much less than that one produced at nervous system level and that the intraluminal ACh release takes place mainly through transmembrane mechanisms and not by exocytosis. As a consequence several doubt emerged regarding the real extracellular effect of that ACh which is released by the non-neuronal cholinergic system localized in the airways. Besides it is coincevable that ACh could display intracellular effects, mainly mediated by intracytoplasmic receptors. On the other hand, ACh degradation capacity in the airways is lower than that in the nervous system, since it is mainly mediated by the BChE enzyme. These data are coherent with a potential paracrine/autocrine loop of ACh on epithelial cells (1,30). Indeed immunohistochemical studies confirm that ACh activity prevails in the nervous fibers among smooth muscles, whereas BChE is directly detectable into smooth muscle cells. Thus, although the mechanisms of ACh degradation in the airways has not been yet completely elucidated, preliminary data allow to hypothesize that, in this setting, lower concentration of released ACh might act through a reverberating loop on cells themselves.
Targets and functions of non-neuronal cholinergic system
The effects of the ACh released through the non-neuronal system can be divided on the bases of the site of release itself, luminal or basal, thus displaying specific effects on the target cells.
Luminal side
When released on the luminal side, ACh can reach a limited number of cells, among which epithelial cells, macrophages and many elements of the immunitary system. Both epithelial cells and macrophages present muscarinic and nicotinic receptors that could interact with the released ACh. In particular the epithelial cells express M1 and M3 receptors and the α and β subunits of the nAChR receptor (31). Through the binding of these receptors, the ACh regulates proliferation of epithelial cells, mucous secretion, and the release of GM-CSF and IL-8, which stimulates ciliary beat. Macrophages express the isoform 3 of the muscarinic receptor and several subunits of the nicotinic one, among which the α9/α10 units (32). M3 receptor activation induces in vitro the release of pro-inflammatory mediators, whereas the stimulation of the the nAChR suppresses macrophages activation with a more general anti-inflammatory effect. It should be noted that, due to their localization, macrophages cannot be reached by the ACh produced by the neuronal cholinergic system and thus identify a specific target of the ACh derived from non-neuronal system and released from the luminal side of epithelial cells.
Latero-basal side
A clear distinction among neuronal and non-neuronal derived ACh is really difficult at cellular basal side. In mouse models, epithelial cells at tracheal level can release ACh following serotonin stimulation thus directly inducing bronchoconstriction; this effect is susceptible to atropine, an inhibitor of muscarinic receptors (33). Few data are currently available on the effect of non-neuronal ACh on smooth muscle cells. It is conceivable that ACh could reach structures close to the epithelium or could be caught by the epithelium itself. Fibroblasts localized in the sub-epithelial lining represent a selective and specific ACh target (34). Below the basal lamina, several immune system and nervous cells can be found. Sensitive and vagal neurons express several nAChR subunits and are so connected to the epithelial cells to be sensitive to inhaled nicotine (35). Their stimulation induces local release of neuropeptides that, by activating local defense response, causes local irritation and provoke cough reflex.
Relevance of non-neuronal cholinergic system in pathogenesis and therapy of airways diseases
Deregulation of muscarinic receptors is a frequent feature of airway diseases such as asthma and COPD, and the use of muscarinic antagonists represents an important strategy in pharmacological treatment of COPD. The contribution of non-neuronal ACh in patho-mechanisms is still partially unclear. A number of data, however, suggests that epithelial ACh may be increased in airway inflammatory diseases, thus contributing to activation of immune cells and to bronchoconstriction. On the contrary, total airway ACh content is reduced in patients affected by cystic fibrosis, and expression of the non-neuronal ACh synthesis and release machinery is down-regulated in acute allergic airway inflammation. The stimulatory effect of ACh on epithelial cell proliferation, the presence of nAChR on airway epithelial cells and the association between use of tobacco and lung cancer clearly suggest the presence of a possible association of the intrinsic epithelial cholinergic system with the development of lung cancer. Small cell lung cancer cells (originating from neuroendocrine cells of the epithelium) and squamous carcinoma cells synthesize and release ACh, and this acts as an autocrine growth factor, operating both via muscarinic M3 and nAChR receptors. Most recently, it has been demonstrated that a variation in a region of 15q25.1 containing nAChR genes coding for subunits α3, α5, β4 is correlated with an increase in lung cancer risk. Nicotinic effects on cell proliferation are summarized in Figure 1. In summary, non-neuronal ACh release is involved in plastic changes and in the activation of the immune response, in ongoing chronic inflammatory airways disease. Furthermore, there is growing evidence that disturbances of this system directly contribute to the development of lung cancer, through stimulation of pro-mitotic activity. In light of the aforementioned evidences, it is reasonable to consider airways non-neuronal cholinergic system as a potential pharmacological target in the treatment of inflammatory and proliferative lung diseases.
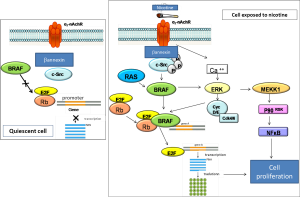
Cholinergic system and lung cancer
Lung cancer is the number one cause of death for solid tumors in Western world and smoking habit is associated to its development in the vast majority of cases. Although the primary mechanism of smoking-induced carcinogenesis is related to smoke’s carcinogens, recent data show that nicotine and nitrosamines bind to nicotinic acetylcholine receptor (nAChR) on lung cancer cells to stimulate tumor growth and inhibit apoptotic death program. Mechanisms of cholinergic signal activation in lung cancer are represented in Figure 2. These experimental observations are consequent to the demonstration that cholinergic system is not only confined in nervous system but, as demonstrated above, is ubiquitous and specifically, present in airways epithelial cells and lung cancer cells. Thus the stimulatory effect of ACh on epithelial cell proliferation, the presence of nACh on airway epithelial cells as well as the exposure to tobacco smoking create a strong rationale to deeper investigate the possible link between non neuronal cholinergic system and susceptibility to lung cancer development. For instance it is well known that small cell lung cancer (SCLC) (36)—which originates from neural crest—and squamous cell carcinoma (SCC) are able to synthetize and produce ACh, which in turn acts as autocrine growth factor, by linking both M3 and nACh receptors (37). nAChR subunit α3β2 mediates smoking related toxic effects through the activation p21, Bcl-2, NF-κB e STAT-1 signaling; the 7α subunit nAChR is involved in damage on cheratinocytes on the oral cavity through the activation of MAP kinases and JAK/STAT pathway (38). Gene expression profiling analyses have been thus addressed to evaluate how tobacco and nicotine can affect receptor expression: in both cases a 7α nAChR-mediated over regulation of cell growth factor and proliferation can be found.
It should be remembered that several allelic variant exist for the vast majority of genes (about 80% of human genome). Genetic polymorphism can be defined as a genetic variation that occurs in more than 1% of a population, whereas a genetic mutation identifies a variation which occur in less than 0.1% of a population. Several types of polymorphisms do exist: the more frequent are single nucleotide polymorphisms (SNPs) which are classified based on their position in the context of the genes: (I) cSNP which are localized in codifying exons and are thus able to induce a variation in the aminoacidic sequence of the protein; (II) pSNPs or peripheral polymorphisms which affect regulatory regions (e.g., promoters, enhancers), introns (splicing regions) and could determine interference which the expressione levels and the structure of the proteins; (III) rSNPs or random polymorphisms, which are detected in intergenic regions (which represent 98.5% of the whole genome) and which have no direct effect on gene expression but could be relevant due to their diagnostic potential according to linkage disequilibrium phenomenon. Indeed SNPs analysis is becoming of great relevance in predictive oncology with the aim to stratify patients based for their risk of cancer onset based on the presence of certain SNPs.
From this perspective, recent studies on lung cancer susceptibility have drawn researchers’ attention on the SNPs of the gene encoding for the nAChR. Interestingly genome-wide sequencing analyses have provided evidences of a significant association between NSCLC risk, smoking behavior and polymorphisms on 15q21 locus, containing genes encoding for nAChR α3, α5 (CHRNA5, CHRNA3 genes) and β4 (CHRNB4 gene) subunits (39-41). In particular a non-synonymous substitution (D398N, substitution of aspartic acid with asparagine in position 398) encoding for a highly conserved region of the receptor (M2 domain) represents one of the most powerful markers of disease risk. That haplotype has been identified among Europeans and Nord Americans, whereas it is really rare among Asiatic and African populations (data from HapMap database, website at www.hapmap.ncbi.nlm.nih.gov). The high SNPs frequency in the 15q21 gene (the rs8034191 e rs1051730 SNPs are detectable in about 50% of Europe population) makes the study of the genes localized in that locus very relevant also in a public health perspective and identifies CHRNA3 e CHRNA5 very promising actionable targets. It has been also documented that SNPs in that genetic regions are related to nicotine dependence; consequently the correlation between lung cancer onset and smoking habit has been also investigated. As expected the risk for disease inset is higher among smokers than in never smokers whereas no correlation has been found within respect to neoplastic histotypes. It is conceivable that 15q21 polymorphisms although not playing a causal role in inducing tumor development (no direct pathogenetic role), might be related to the induction of smoking habit, which, in turn, is the most relevant risk factor for lung cancer. Thus, genetic variability of the long arm of chromosome 15 is directly related to nicotine dependence and consequently, might expose to the risk of smoke-related disease, among which the most important is lung cancer (39).
Overall these findings show how genetic interindividual variability plays a central role in the pathogenesis of complex or polygenic diseases, among which cancer, by modulating the mechanisms by which each subject reacts to external stimuli (e.g., nicotine exposure by regulating ligand-receptor affinity) and by affecting inclination towards given environmental stimuli (e.g., persistence of smoking habit despite its predictable consequences). From this perspective, nAChRs are becoming interesting targets both in lung cancer screening and in molecular tailored therapy. It has been demonstrated that in SCC the cholinergic signaling is up-regulated and in this scenario, nicotine exposure can activate different oncogenic pathways, being responsible for tumor spread and neoplastic angiogenesis. Thus pharmacological inhibition of cholinergic receptors—both nicotinic and muscarinic—might be a promising tool to limit basal and nicotine-stimulated tumor growth.
Molecular mechanisms of cholinergic signal in lung cancer
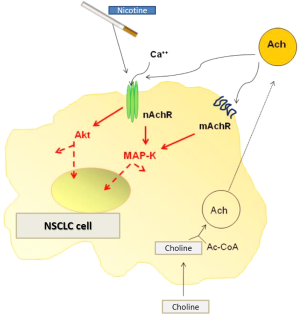
According to what has been previously described, correlation between lung cancer onset and nicotine depends on two different mechanisms: (I) inter-individual genetic variability (polymorphisms in the locus 15q21) which is responsible for a higher susceptibility or predisposition to the onset of lung cancer, mainly due to an increased nicotine dependence; (II) the proliferative and anti-apoptotic effect on neoplastic cells played directly by nicotine through cholinergic receptors activation. Historically the first study which has shown a nicotine effect different from neuronal signaling is that of Schuller and coll (42). The authors demonstrated a proliferative effect mediated by nicotine in a series of lung cancer cell lines, through the increased release of growth factors (VEGF, HGF, TGF-β, PDGF, TGF-α) and their receptors (VEGFR, MET, EGFR). In detail, nicotine induced EGFR transactivation through the increase of intacellular calcium which is responsible for the activation of several kinases downstream EGFR. It has been reported that in gastric cancer nicotine is able to induced the overexpression of VEGFR through the activation of COX-2; the latter induce an increase in neoplastic angiogenic and invasion capacity which involves some elements of extracellular matrix, such as metalloproteases (MMP2 and MMP-9) and enzymes responsible for plasminogen activation cascade. In lung adenocarcinoma cell line A549 the exposure to nicotine promotes inhibition of phosphorylation of the protein phosphatase 1 (PP1) which in turn, induces the deregulation of protein p27 Kip1. The latter plays inhibitory effects on the cyclin-dependent kinase 1 which determines cell cycle progression. Moreover nicotine is responsible for an increased NSCLC cell proliferation through the expression of fibronection and α5β1-integrin and the activation of ERK and PI3K-mTOR signaling. More recent studies have demonstrated that the nAChR is the main mediator of proliferative effects of nicotine of transformed cells. Consequently the 7α subunit identifies a novel druggable target of NSCLC therapeutic approach. Growing evidence demonstrates similar effects of the subunit 7α nAChR in SCLC and mesothelioma as well. The activation of the nicotinic receptor induces an increase in cell proliferation and survival mediated by the MEKK-1, ERK1/2 e p90RSK kinases; notably in A549 cells the nicotine-induced activation of the MPAKs occurs in a 7α nAChR-mediated manner (43). Moreover biological effects of nicotine on transformed cells involves other mediators and transcription factors such as, NF-κB, Src, Akt, HIF-1 and the lipo-oxygenase cascade, as well (44). Nicotine improves cancer (NSCLC, SCLC, breast and ovary cancer) cells survival by inducing avoidance of apoptosis mediated by a number of stimuli (e.g., radiation, chemotherapy agents, oxidative stress). Besides nicotine promotes tumor progression and spreading since it can induce angiogenesis and arteriogenesis; tissue hypoxia and ischemia themselves induce overexpression and sensitization of endothelial cells to the 7α subunit of nAChR (45). Thus the nAChR plays a crucial role in the complex molecular network which is responsible for tumor progression orchestrated by angiogenic processes.
It has been recently reported in lung tumor samples (SCC) the presence of high levels of α5 e β3 nAChR mRNA, in association to high levels of ACh consequent to an increase of ChAT due to low expression of the cholinesterase enzyme. These findings demonstrate that in lung cancer the cholinergic signal is aberrantly activated, with increased receptors levels and lower levels of their inhibitors. Similar results have been reported in vitro through the exposure to nicotine of lung cancer cell line (H520) and by measuring the receptor activity levels. The results demonstrated that NSCLC expresses the ACh signaling and that both ACh and nicotine may activate the cascade thus promoting tumor growth. In such setting, cell exposure to cigarette smoking represents on one hand a relevant stimulus to cancer cell proliferation and on the other a novel actionable target. From this perspective it has been shown that pharmacologic block of M3 muscarinic receptor with darifenacin can inhibits in vitro MAPs-mediated cell proliferation induced by activation of muscarinic and nicotinic receptors (46) MAPKs activation is the key point of the signaling cascade activated by the two receptor families and the blockade of the M3 receptors could represent ideally a novel potentally targetable axis in lung cancer therapy platform.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol 2008;130:219-34. [PubMed]
- Wessler I, Kirkpatrick CJ, Racké K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther 1998;77:59-79. [PubMed]
- Sleigh MA. Ciliary function in mucus transport. Chest 1981;80:791-5. [PubMed]
- Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther 2007;115:208-22. [PubMed]
- Cho T, Chan W, Cutz E. Distribution and frequency of neuro-epithelial bodies in post-natal rabbit lung: quantitative study with monoclonal antibody against serotonin. Cell Tissue Res 1989;255:353-62. [PubMed]
- Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol 2007;10:419-35. [PubMed]
- De Proost I, Pintelon I, Brouns I, et al. Functional live cell imaging of the pulmonary neuroepithelial body microenvironment. Am J Respir Cell Mol Biol 2008;39:180-9. [PubMed]
- Reid L, Meyrick B, Antony VB, et al. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med 2005;172:136-9. [PubMed]
- Krasteva G, Kummer W. “Tasting” the airway lining fluid. Histochem Cell Biol 2012;138:365-83. [PubMed]
- Pfeil U, Lips KS, Eberling L, et al. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am J Respir Cell Mol Biol 2003;28:473-7. [PubMed]
- Racké K, Matthiesen S. The airway cholinergic system: physiology and pharmacology. Pulm Pharmacol Ther 2004;17:181-98. [PubMed]
- Barnes PJ. Distribution of receptor targets in the lung. Proc Am Thorac Soc 2004;1:345-51. [PubMed]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 2008;154:1558-71. [PubMed]
- Ramsay RR, Naismith JH. A snapshot of carnitine acetyltransferase. Trends Biochem Sci 2003;28:343-6. [PubMed]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007;24:1227-51. [PubMed]
- Falk-Vairant J, Meunier FM, Lesbats B, et al. Cell lines expressing an acetylcholine release mechanism; correction of a release-deficient cell by mediatophore transfection. J Neurosci Res 1996;45:195-201. [PubMed]
- Dunant Y, Cordeiro JM, Gonçalves PP. Exocytosis, mediatophore, and vesicular Ca2+/H+ antiport in rapid neurotransmission. Ann N Y Acad Sci 2009;1152:100-12. [PubMed]
- Malo M, Israël M. Expression of the acetylcholine release mechanism in various cells and reconstruction of the release mechanism in non-releasing cells. Life Sci 2003;72:2029-38. [PubMed]
- Fukami T, Yokoi T. The emerging role of human esterases. Drug Metab Pharmacokinet 2012;27:466-77. [PubMed]
- Pfeil U, Lips KS, Eberling L, et al. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am J Respir Cell Mol Biol 2003;28:473-7. [PubMed]
- Fisher AB, Dodia C, Chander A, et al. Transport of choline by plasma membrane vesicles from lung-derived epithelial cells. Am J Physiol 1992;263:C1250-7. [PubMed]
- Wang T, Li J, Chen F, et al. Choline transporters in human lung adenocarcinoma: expression and functional implications. Acta Biochim Biophys Sin (Shanghai) 2007;39:668-74. [PubMed]
- Lips KS, Volk C, Schmitt BM, et al. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 2005;33:79-88. [PubMed]
- Eiden LE. The cholinergic gene locus. J Neurochem 1998;70:2227-40. [PubMed]
- Reinheimer T, Bernedo P, Klapproth H, et al. Acetylcholine in isolated airways of rat, guinea pig, and human: species differences in role of airway mucosa. Am J Physiol 1996;270:L722-8. [PubMed]
- Wessler I, Michel-Schmidt R, Brochhausen C, et al. Subcellular distribution of choline acetyltransferase by immunogold electron microscopy in non-neuronal cells: placenta, airways and murine embryonic stem cells. Life Sci 2012;91:977-80. [PubMed]
- Klapproth H, Reinheimer T, Metzen J, et al. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 1997;355:515-23. [PubMed]
- Song P, Sekhon HS, Jia Y, et al. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 2003;63:214-21. [PubMed]
- Horvath G, Mendes ES, Schmid N, et al. The effect of corticosteroids on the disposal of long-acting beta2-agonists by airway smooth muscle cells. J Allergy Clin Immunol 2007;120:1103-9. [PubMed]
- Proskocil BJ, Sekhon HS, Jia Y, et al. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology 2004;145:2498-506. [PubMed]
- Moulton BC, Fryer AD. Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br J Pharmacol 2011;163:44-52. [PubMed]
- Koarai A, Traves SL, Fenwick PS, et al. Expression of muscarinic receptors by human macrophages. Eur Respir J 2012;39:698-704. [PubMed]
- Kummer W, Wiegand S, Akinci S, et al. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res 2006;7:65. [PubMed]
- Pieper MP, Chaudhary NI, Park JE. Acetylcholine-induced proliferation of fibroblasts and myofibroblasts in vitro is inhibited by tiotropium bromide. Life Sci 2007;80:2270-3. [PubMed]
- Dehkordi O, Kc P, Balan KV, et al. Airway-related vagal preganglionic neurons express multiple nicotinic acetylcholine receptor subunits. Auton Neurosci 2006;128:53-63. [PubMed]
- Song P, Sekhon HS, Jia Y, et al. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 2003;63:214-21. [PubMed]
- Song P, Spindel ER. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J Pharmacol Sci 2008;106:180-5. [PubMed]
- Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 2008;29:151-8. [PubMed]
- Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 2008;40:616-22. [PubMed]
- Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008;452:633-7. [PubMed]
- Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008;452:638-42. [PubMed]
- Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol 1998;55:1377-84. [PubMed]
- Zheng Y, Ritzenthaler JD, Roman J, et al. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol 2007;37:681-90. [PubMed]
- Zhang Q, Tang X, Zhang ZF, et al. Nicotine induces hypoxia-inducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clin Cancer Res 2007;13:4686-94. [PubMed]
- Grozio A, Paleari L, Catassi A, et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anti-cancer drug development. Int J Cancer 2008;122:1911-5. [PubMed]
- Song P, Sekhon HS, Fu XW, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 2008;68:4693-700. [PubMed]


