Correlation in histological subtypes with high resolution computed tomography signatures of early stage lung adenocarcinoma
Introduction
Lung cancer is the most frequently diagnosed cancer and remains a leading cause of cancer death worldwide (1). While lung adenocarcinoma is the most common histological subtype (2). In 2011 Feb, International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) jointly published a newly lung adenocarcinoma classification. The classification addresses both resection specimens and small biopsies/cytology. New concepts such as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) were added. And invasive adenocarcinomas (IA) were classified with lepidic, acinar, papillary, micropapillary, and solid patterns Travis et al. (3). According to this new classification, patients who have AIS, MIA, and lepidic predominant adenocarcinomas have shown excellent survival rates after complete resection.
The National Lung Screening Trial (NLST) found a relative reduction in mortality from lung cancer with low-dose computed tomography (CT) screening of 20.0% compared with chest radiography (4). While on CT scans, these indolent and less aggressive tumors of AIS, MIA, and lepidic predominant adenocarcinomas frequently present as pure ground-glass opacity (pGGO) or mixed ground-glass opacity (mGGO) (5). Several other studies confirmed a well correlation between CT findings and histologic prognostic factors in lung adenocarcinomas (5-7). However, when pGGOs are greater than 15 mm in diameter or have high pixel attenuation (>−472 HU), the nodules are more likely to be IA (8). Recently, a similar observation has been documented for early stage tumors about GGO component. They found that in patients with tumors smaller than 3 cm, disease free survival (DFS) was significantly associated with solid tumor size, but not with whole tumor size (9).
GGO proportion is very important for prognosis in lung adenocarcinoma. Previous studies have explored CT features correlating with pathological invasiveness. They reported that tumors with higher solid volume proportion and larger diameter indicated IA rather than non-invasive lesions (AIS and MIA) (10-12). Nevertheless, uncertainty remains on the correlation between image characteristics of the nodules in CT scans and adenocarcinoma histopathologic subtypes.
We performed this retrospective analysis to estimate the correlation between preoperative high resolution computed tomography (HRCT) scan and postoperative histopathology of stage IA lung adenocarcinoma in East Asian Chinese population. In addition, we’d like to find some independent risk factors in HRCT signatures which can help distinguish histological subtypes in early stage lung adenocarcinoma to predict their prognosis.
Methods
Patients
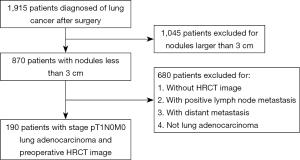
We retrospectively reviewed the clinical records and CT images of 190 patients (106 female and 84 male; age range, 29–81 years; average age, 59 years) with resected, preoperatively untreated pT1N0M0 stage IA adenocarcinomas. These patients underwent lung cancer surgery in Jinling hospital (Jiangsu, China) between July 2008 and March 2015. All cases met the 2011 IASLC/ATS/ERS classification (3) of lung adenocarcinomas and were considered as stage IA according to the 7th Edition Union for International Cancer Control/American Joint Committee on Cancer TNM classification (13). Patients concurrent with other tumors were excluded (Figure 1).
Histological evaluation
Histological classification was according to the IASLC/ATS/ERS classification of lung adenocarcinomas (3). Tumors were classified as atypical adenomatous hyperplasia (AAH), AIS, MIA and IA. IA was further divided into lepidic predominant, acinar predominant, papillary predominant, micropapillary predominant, solid predominant with mucin, invasive mucinous adenocarcinoma and colloid predominant.
HRCT evaluation
Two radiologists with 3 years of experience retrospectively interpreted the HRCT images independently. If they have different opinions, a third radiologist will confirm it. Margin characteristics of nodules and the internal characteristics within the nodules were all analyzed. These characteristics included diameter, proportion of GGO, margin, border definition, bubble lucency, shape, air bronchogram, vessel convergence sign, pleural retraction, pleural thickening, lymphadenopathy, enhancement and so on. GGO was defined as hazy and amorphous increased lung attenuation without obscuration of the underlying vascular markings and bronchial walls. In regard to evaluated the GGO proportion of the tumor (GGO/tumor ratio; G/T ratio), the G/T ratio was calculated as (1-DSOL)/ DGGO, where DGGO was the largest area of the tumor, and DSOL was the largest solid area of the tumor (14).
Statistics
Statistical analysis was performed using the one-way ANOVA for continuous variables and chi-square test for categorical variables. To analyze the relationship between HRCT and histological subtypes, we used logistic regression model for multivariate analysis. Values of P<0.05 were considered significant. Statistical analysis was conducted using Statistical Product and Service Solutions (SPSS) version 20.0.
Results
Clinical characteristics
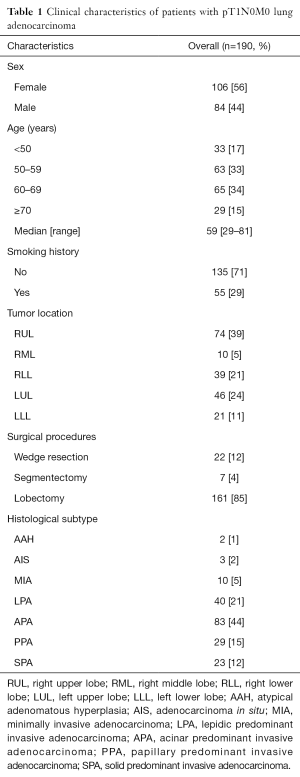
Clinical features including sex, age, smoking history, tumor location, surgical procedures, and histological subtype were summarized in Table 1. Most patients were female (56%) and never smoking (71%). Median age was 59 years (range, 29–81 years). A large majority of tumors (65%) were located in right lung. Seventy four tumors (39%) were located in right upper lobe (RUL) and 46 tumors (24%) were located in left upper lobe (LUL). One hundred and sixty one patients (85%) underwent lobectomy, 22 patients (12%) underwent wedge resection, and 7 patients (4%) underwent segmentectomy (Table 1). No patient received preoperative adjuvant chemotherapy and/or radiation therapy. Among the patients, 55 received EGFR mutation detection, 32 (58%) of them were EGFR mutation positive (Table 2).
Full table
Full table
Histological characteristics
According to the IASLC/ATS/ERS classification, there were 2 AAH cases (1%), 3 AIS cases (2%), 10 MIA cases (5%) and 175 IA cases (92%) which were mostly acinar predominant adenocarcinoma (44%) (Table 1).
Since solid adenocarcinomas have a poor prognosis, papillary and acinar adenocarcinomas have an intermediate prognosis, and lepidic adenocarcinomas have a favorable prognosis (15). In order to better carry out statistics, we classified lung adenocarcinoma in four groups. Lepidic group contains AAH, AIS, MIA and lepidic predominant IA. Acinar group is acinar predominant IA. Papillary group is papillary predominant IA. And solid group is solid predominant invasive adenocarcinoma (SPA).
Univariate analysis
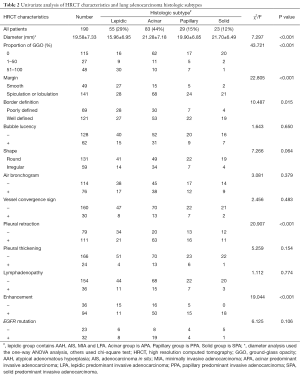
We conducted one-way ANOVA analysis to find the differences among four groups. Median tumor diameter was 19.58±7.33 mm, and it was significantly lower in level 1 group (15.96±6.95 mm), whereas acinar predominant adenocarcinoma and papillary-predominant (21.28±7.18 mm; 19.90±6.85 mm, respectively) were of intermediate size, and level 4 (21.70±6.49 mm) were larger (P<0.001). GGO proportion (P<0.001), margin (P<0.001), border definition (P=0.015), pleural retraction (P<0.001) and enhancement (P<0.001) had statistically significant differences in four histological levels (Table 2). There were no significant differences in bubble lucency, shape, air bronchogram, vessel convergence sign, pleural thickening, lymphadenopathy and EGFR mutation by chi-square test (Table 2).
Multivariate analysis
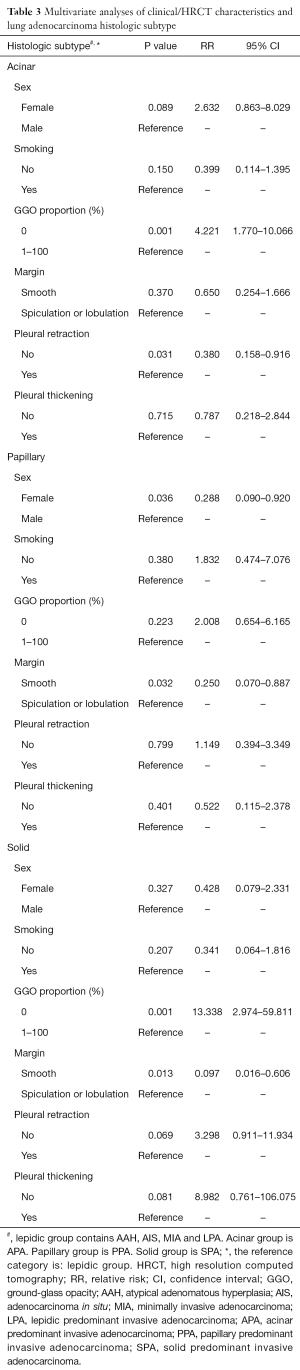
To find the association between HRCT characteristics and histological subtypes, we conducted the multivariate analysis referenced for lepidic group which indicated that GGO proportion and pleural retraction were independent associated with acinar group (RR=4.221, 95% CI: 1.770–10.066, P=0.001; RR=0.380, 95% CI: 0.158–0.916, P=0.031, respectively, Table 3). Tumors which HRCT characterized without GGO component and with positive pleural retraction were more likely to be acinar predominant IA than lepidic predominant IA. While male and whose nodule margin with spiculation or lobulation were prone to papillary predominant IA (RR=0.288, 95% CI: 0.090–0.920, P=0.036; RR=0.250, 95% CI: 0.070–0.887, P=0.032, respectively, Table 3). GGO proportion and nodule margin were independent prediction factors in solid group (RR=13.338, 95% CI: 2.974–59.811, P=0.001; RR=0.097, 95% CI: 0.016–0.606, P=0.013, respectively, Table 3). Nodules without GGO component or nodule margin with spiculation or lobulation were risk factors for poor prognosis histological subtypes.
Full table
Discussion
In this study, we retrospectively reviewed 190 patients with preoperatively untreated pT1N0M0 stage IA adenocarcinomas in East Asian Chinese population, and investigated a number of prediction factors in clinical and HRCT scan between different histological subtypes.
In univariate analysis, we found that smaller tumor size and larger GGO proportion were significantly associated with indolent and less aggressive tumors. Similarly, there were several studies also found that the size and mass of the nodule are determinants of invasive adenocarcinoma (8,16-18). While, recently an observation found that in patients with tumors smaller than 3 cm, DFS was significantly associated with solid tumor size, but not with whole tumor size in early stage tumors (9). They suggested that nomogram-based T descriptors provide better prediction of survival than conventional T descriptors (9). Our results thus are in concordance with previous findings and support the hypothesis that larger tumor size and smaller GGO proportion are risk factors for poor prognosis histological subtypes in early stage lung adenocarcinomas. In addition, we have shown that tumors with spiculation or lobulation, poorly defined border and positive pleural retraction were more likely to be poor prognosis histological subtypes. Not all patients took enhanced CT examination, but tumors with enhancement were significantly relevant with acinar predominant IA.
However, we did not find significant difference in bubble lucency, shape, air bronchogram, vessel convergence sign, pleural thickening, lymphadenopathy in EGFR mutation (Table 2). While, different observations were found last year (19,20). One of the studies enrolled 35 patients with 72 lesions. Among them, 33 (45.8%) tumor lesions were found harboring EGFR mutations, and their founding indicated that there was a high discrepancy of driver mutations in NSCLC patients with ground-glass nodules (GGNs) (19). Another research showed that EGFR mutation especially L858R was detected more frequently in invasive solid pattern and significantly less in pure GGO pattern in stage I lung adenocarcinoma (21). In our study, 55 patient conducted EGFR detection, only 9 of them were pGGOs. Although there was no significant difference, a majority of patients with positive EGFR mutation were acinar predominant IA (59%) (Table 2).
In multivariate analysis, we used lepidic group (histological subtypes with favorable prognosis) as reference for logistic regression model. Tumors which HRCT characterized without GGO component and with positive pleural retraction were more likely to be acinar predominant IA than lepidic predominant IA. Male and whose nodule margins with spiculation or lobulation were prone to papillary predominant IA. Solid nodules without GGO component or nodule margin with spiculation or lobulation were prediction factors for poor prognosis histological subtypes.
Our results suggest that GGO proportion, margin signature and pleural retraction should be focused initial evaluation of histological subtypes in early stage lung adenocarcinomas. Several studies also showed that proportion of GGO remains important for predicting less invasive lung cancer (22-24). In their opinion, small peripheral adenocarcinoma or BAC may present with a high ratio of GGO components on CT scans. Investigators have reported that the solid components in advanced-stage lesions are significantly larger than those in lesions at earlier stage (5,25). Solid component increases the level of suspicion for invasive adenocarcinoma. Our study firstly evaluated the correlation between HRCT image and four histological subtypes.
However, we did not find significant difference in smoking history (Table 3). Recent studies have showing that smoking was a significant predictive for unfavorable prognosis in lung adenocarcinoma (26). And it was robustly associated with GGO growth (27). In our study, most patients were female (56%) (Table 1). We could not exclude passive smoking and exposure to dust (28) from those without smoking history. Besides, air pollution may be another reason.
This study had some limitations. First, this was a retrospective study and the number of patients was relatively small for our strict inclusion criteria. And some cases were excluded for not available HRCT image in other hospitals. We hope to further validate these results in a prospective study with a large number of cases. Second, since our study reviewed patients between July 2008 and March 2015, we could not get complete prognostic information now. We look forward to confirm these results and find more useful risk factors in early stage lung adenocarcinoma prognosis by follow-up research.
In conclusion, we demonstrated that nodules with spiculation or lobulation and less GGO proportion are determinants of poor prognosis histological subtypes in stage IA lung adenocarcinoma patients according to the 2011 histologic IASLC/ATS/ERS classification. HRCT signatures such as tumor diameter, GGO proportion, margin, border definition and pleural retraction may help infer histological subtypes of lung adenocarcinoma in early stage.
Acknowledgements
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province (No. BK2011658), Clinical Science and Technology Project of Jiangsu Province (No. BL2013026) and The National Natural Science Foundation of China (No. 81302032).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Ethical Committee (approval number: 2016NZHX-005) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973-2002. Cancer Epidemiol Biomarkers Prev 2007;16:2724-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kuriyama K, Seto M, Kasugai T, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol 1999;173:465-9. [Crossref] [PubMed]
- Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001;220:803-9. [Crossref] [PubMed]
- Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 2011;26:106-18. [Crossref] [PubMed]
- Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol 2014;202:W224-33. [Crossref] [PubMed]
- Song SH, Ahn JH, Lee HY, et al. Prognostic impact of nomogram based on whole tumour size, tumour disappearance ratio on CT and SUVmax on PET in lung adenocarcinoma. Eur Radiol 2016;26:1538-46. [Crossref] [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [Crossref] [PubMed]
- Eguchi T, Yoshizawa A, Kawakami S, et al. Tumor size and computed tomography attenuation of pulmonary pure ground-glass nodules are useful for predicting pathological invasiveness. PLoS One 2014;9:e97867. [Crossref] [PubMed]
- Dai J, Shi J, Soodeen-Lalloo AK, et al. Air bronchogram: A potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer 2016;98:22-8. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Hong SJ, Kim TJ, Choi YW, et al. Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes. Eur Radiol 2016;26:3660-8. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Isaka T, Yokose T, Ito H, et al. Comparison between CT tumor size and pathological tumor size in frozen section examinations of lung adenocarcinoma. Lung Cancer 2014;85:40-6. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 2014;145:464-72. [Crossref] [PubMed]
- Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. [Crossref] [PubMed]
- Yang Y, Yang Y, Zhou X, et al. EGFR L858R mutation is associated with lung adenocarcinoma patients with dominant ground-glass opacity. Lung Cancer 2015;87:272-7. [Crossref] [PubMed]
- Hsu KH, Chen KC, Yang TY, et al. Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 2011;6:1066-72. [Crossref] [PubMed]
- Matsuguma H, Oki I, Nakahara R, et al. Comparison of three measurements on computed tomography for the prediction of less invasiveness in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;95:1878-84. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [Crossref] [PubMed]
- Tamura M, Shimizu Y, Yamamoto T, et al. Predictive value of one-dimensional mean computed tomography value of ground-glass opacity on high-resolution images for the possibility of future change. J Thorac Oncol 2014;9:469-72. [Crossref] [PubMed]
- Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. [Crossref] [PubMed]
- Tomita M, Ayabe T, Chosa E, et al. Impact of smoking on outcome of resected lung adenocarcinoma. Gen Thorac Cardiovasc Surg 2015;63:608-12. [Crossref] [PubMed]
- Kobayashi Y, Sakao Y, Deshpande GA, et al. The association between baseline clinical-radiological characteristics and growth of pulmonary nodules with ground-glass opacity. Lung Cancer 2014;83:61-6. [Crossref] [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics 2006;26:59-77. [Crossref] [PubMed]


