Immunotherapy and radiation therapy for operable early stage and locally advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer mortality in the United States. About 25% of patients with non-small cell lung cancer (NSCLC) present with early stage disease, which is potentially curable with standard of care lobectomy (1,2). Local control is generally excellent after surgery or radiation. Stereotactic body radiotherapy (SBRT) has become an excellent alternative treatment option in patients with early-stage, node negative disease (3). Local control is about 90% at 3 years. Early data in patients with operable patients treated with SBRT indicate local control is 92% and 73% at 5 years for T1 and T2 disease, respectively (4).
For patients with node-positive or locally advanced operable disease, conventionally fractionated radiation therapy (RT) can be integrated in several different ways. In resectable locally advanced patients, typically stage IIIA (AJCC v7), primary surgery is performed before or after platinum-based chemotherapy, and post-operative RT is indicated in disease with persistent N2 lymph nodes. Preoperative chemoradiotherapy is an alternative approach to the treatment of resectable N2 disease. Patients with unresectable locally advanced NSCLC are treated with curative intent concurrent or sequential chemoradiation.
However, there is a critical need to develop better therapeutic approaches to treat patients with early and locally advanced stage disease and to integrate systemic therapies that have the capacity to effectively eradicate micrometastatic disease and create a sustained systemic response.
Patients with early stage disease still have high risk of relapse
Although local control is high for patients with operable NSCLC, systemic relapse remains the predominant failure pattern. Even among patients with the earliest clinical stage of lung cancer, 50% will die within 5 years of diagnosis after lobectomy (5). For patients with Stage II and IIIA disease and good performance status, platinum-based chemotherapy is recommended to improve systemic relapse rates.
There exists a subset of early stage patients with identifiable poor prognostic characteristics. This includes patients with a suboptimal gene profile, and a number of variably expressed tumor markers and oncogenes (6,7). In one analysis, survival at 5 years in low risk Stage I patients was nearly 90%, but in high risk patients survival was nearly 40% (8). An example of a risk factor is histologic subtype of lung adenocarcinoma, where certain growth patterns such as solid or micropapillary indicate poorer prognosis (9-11). We and others have previously reported that increased SUVmax on pre-treatment fluorodeoxyglucose-PET (FDG-PET) correlate with poorer local control and survival after treatment with SBRT, consistent with surgical series (12). Similar to operable patients, unfavorable subsets of patients have been identified in inoperable patients that are at high risk for nodal and distant failures. These are based on tumor and treatment-related characteristics such as age, functional status, tumor size, histology, proximity to the hilum, and deliverable radiation dose (13).
The opportunity to combine immunotherapy and radiation
There exists a growing body of evidence that T-cell checkpoint inhibitors have robust and enduring activity in some patients with metastatic lung cancer (14-17). Approximately 20% of patients with previously treated lung cancers have objective response to anti-PD-1 or anti-PD-L1 therapies. The responses may be remarkably durable and the treatment associated with good tolerability. Thus far in patients with lung cancers these studies have largely been in patients with metastatic disease, but T-cell checkpoint inhibitors in melanomas have been shown to improve relapse free survival compared to placebo (HR 0.75, P=0.0013) (18). There is a critical unmet need to translate the potential benefits of T-cell checkpoint inhibitors into the early-stage setting for patients with lung cancers. Additionally, as it is only a subset of patients who appear to benefit from anti-PD-1 or anti-PD-L1 therapies, there is also a need to identify effective combination approaches that can augment the benefit of immunotherapy for patients (19,20).
In this context, the opportunity to combine immunotherapy and RT represents a unique approach toward several key challenges in the treatment of patients with lung cancers: (I) can immunotherapy be integrated with RT to improve systemic relapse in patients with early stage lung cancers treated with surgery? (II) can RT in combination with immunotherapy be performed safely and can a synergistic, appropriately sequenced combination be determined?
An analogy to this potential relationship exists in the role of concurrent chemoradiation. In multiple solid tumors, including NSCLC, head and neck disease, and gynecologic malignancies, combined modality therapy with RT and chemotherapy is more effective than either alone, and even more effective compared to sequential therapy. This results in both increased local and systemic control (21). Combined modality therapy has been extensively studied as modulating tumor-host interactions and may improve treatment beyond simply radiosensitization of tumor cells.
Immunotherapy exists in many forms including adoptive T-cell transfer, oncolytic viruses, and cytokine therapy, among other modalities. Currently, immunotherapy using immune checkpoint inhibitors has offered unprecedented rates of response and has since attracted intense attention. Thus the focus of this review will primarily be on immune checkpoint inhibitors and their combination with RT in operable NSCLC.
The activity of immunotherapy in inoperable and metastatic NSCLC
Over 50% of patients with NSCLC will present with metastatic disease and will be treated with chemotherapy with or without local palliative RT. Approximately 18% of patients will present with Stage IIIB unresectable disease and will go on to have chemoradiation with curative intent (22). In these populations, immunotherapy, despite representing an already heavily treated and frail population with a guarded prognosis, has yielded significant improvements in overall survival (20).
The activity of pembrolizumab was reported in a Phase 1 study that analyzed both efficacy and safety (20). After treatment with pembrolizumab, the objective response rate was 19.4%, and the median duration of overall survival was 12 months. In patients with PD-L1 expression in at least 50% of tumor cells, the objective response rate was 45.2% and median overall survival was not reached. The recently published study of first-line pembrolizumab showed it was superior to chemotherapy in NSCLC patients without sensitizing EGFR mutations or ALK translocations that express the protein PD-L1 in more than 50% of cells (23).
Multiple other studies have shown activity of these and other immune checkpoint inhibitors in NSCLC, which has led to several receiving FDA approval for use in first-line or progressive disease (Hellman ASCO 2016) (24,25). These data also indicate there likely exist tumor-specific characteristics to guide whom may best respond to these therapies.
Immune editing with radiation and improved local control when combined with immune therapy in solid tumors
After treatment, tumor cell transformation drives activation of the host immune response, with modifications in both the innate and adaptive systems. A growing body of evidence suggests that RT can quantitatively augment the immune system by directly upregulating tumor-associated antigens (TAAs), augment MHC class I surface expression in a dose-responsive manner, and increase T-cell tumor-specific CD8+ T cells (26,27). Some tumors downregulate MHC expression to evade immune detection, but upregulation characteristics after RT exposure may prevent this.
Beyond MHC class I surface expression, RT may also stimulate the immune system via activating dendritic cells and increasing antigen cross-presentation. This also increases FAS surface expression, which, in turn, induces programmed cell death. FAS is a cell surface receptor that leads to programmed cell death. FAS upregulation ultimately increases the density of tumor-infiltrating lymphocytes, and upregulates PD-L1 expression (28,29). RT already is known to generate inflammation, increase antigen presentation, and modify the tumor microenvironment. In multiple patient reports, the stimulatory effect of RT inducing an abscopal effect (even in the absence of immunotherapy) has been shown using a variety of RT total doses and fractionation schemes (30).
Multiple preclinical studies show that checkpoint blockade augments the immunostimulatory effects of RT to improve local disease control. Demaria et al. showed in breast cancer cell lines that anti-CTLA4 therapy sensitized cells to RT (31). This was additionally demonstrated in an orthotopic glioblastoma model when combining anti-CTLA4 with stereotactic radiosurgery (SRS), a highly potent local therapy. SRS plus checkpoint blockade improved overall survival by 50% as a product of improved local control (32). RT has been shown in melanoma to augment the immune environment leading to the abscopal effect after combination RT and immunotherapy using anti-CTLA4 therapy (33,34). Deng et al. first showed that RT upregulated PD-L1, and then showed that anti-PD-L1 therapy enhanced the therapeutic efficacy of ionizing RT. This was primarily accomplished through an enhanced cytotoxic T-cell dependent mechanism. This combined approach also reduced the accumulation of tumor-infiltrating suppressor cells (35). Sharabi et al. showed that anti-PD1 therapy, when combined with stereotactic RT in mouse models of melanoma or breast cancer, increased T-cell infiltration into tumor and enhanced antigen presentation in draining lymph nodes (36).
Combined therapy leading to abscopal and sustained systemic response
While the above mentioned studies suggest that RT and immunotherapy may work synergistically to improve local control, but the clinically unmet need even in patients with localized disease is to improve systemic control given the high propensity for distant progression. Deng et al. in the same study as above showed both an abscopal effect and a sustained anti-tumor effect after combined therapy. Using a TUBO breast cancer model, mice receiving both anti-PD1 therapy and irradiation of a single lesion showed abscopal effect by growth rate reduction of a second unirradiated tumor. After complete tumor eradication, mice were rechallenged with the same tumor and no palpable tumors developed on the dual-treated mice (35). Park et al. showed a similar result with melanoma and renal cancer mouse models; however, they further showed that the increased antigenicity was tumor-specific when mice bore both tumors (37).
Postow et al. described a patient with metastatic melanoma who was treated with paraspinal SBRT and anti-CTLA4 therapy and who was later found to have a decrease in non-irradiated splenic and hilar masses (34). Golden et al. found a similar effect when a patient with NSCLC was treated with combined therapy. This patient received liver SBRT for a NSCLC metastasis and anti-CTLA4. Not only did the irradiated lesion improve, but there was also significant improvement in nonirradiated disease in the lung, skeleton, and elsewhere in the liver (38).
Multiple other clinical reports show the abscopal effect in patients who have received combined RT and immunotherapy. In one series on the combination of anti-CTLA4 and RT, there was a range of 3–6 months from after treatment until an abscopal effect was reached. A range of 5–47 months was observed from the occurrence of the abscopal effect until further disease progression (39). It is important to note that there was significant heterogeneity in tumor type, site irradiated, and total dose and fractionation of RT. The optimal RT regimen, dose and fractionation to elicit an abscopal effect in combination with immunotherapy remain indeterminate.
Neoadjuvant or adjuvant immunotherapy studies show promise
Several clinical studies have shown activity of various forms of neoadjuvant immunotherapy, including adoptive cell transfer, vaccines, and tumor necrosis factor (TNF) therapies. A study with neoadjuvant chemo-immunotherapy in Stage IIB–IIIB NSCLC patients used cisplatin and gemcitabine, and then randomized patients to concurrent recombinant TNF fused with thymosin-alpha. Seventy-one percent had response to chemo-immunotherapy, versus 50% to neoadjuvant chemotherapy alone (Lazutin ASCO 2015). In an updated analysis, the chemotherapy alone group showed a decrease in NK cells while the chemo-immunotherapy group did not (Zlatnik ASCO 2016).
Kimura et al. performed a randomized study of adjuvant chemo-immunotherapy versus immunotherapy alone in patients with IB–IV NSCLC after thoracotomy. Patients who received non-curative resections were included. Chemotherapy was given in platinum-doublets, and immunotherapy consisted of activated killer T-cells and dendritic cells. There was a remarkable difference in 5-year overall survival after the addition of immunotherapy after surgery (81.4% vs. 48.3%, HR 0.229, P=0.0013). In addition, there was improvement in recurrence-free survival (40). A meta-analysis of 4 randomized trials consisting of 472 patients showed a significant benefit of adjuvant adoptive immunotherapy with a 39% relative reduction in risk of death. Two of the 4 studies allowed RT as part of treatment (41).
The Radiation Therapy Oncology Group (RTOG) proposed a Phase II study of adjuvant immunotherapy and RT in patients with completely resected Stage II and IIIA NSCLC (RTOG 9909, ClinicalTrials.gov number: NCT00006470). Patients received surgery and within 7 weeks, began two anti-idiotype vaccines (one which mimicked CEA, and the other mimicked the human milk fat globule antigen) and 50.4 Gy in 28 fractions of RT. These vaccines are used to mimic TAAs. Proposed accrual was 54 patients; however, only 22 patients were accrued and the study closed without reporting results.
Current clinical studies in operable patients
There are multiple current clinical trials open exploring neoadjuvant and adjuvant immunotherapy in operable NSCLC, with a significant focus on checkpoint inhibitors given promising results in patients with advanced or metastatic disease. There is significant heterogeneity in the type of immunotherapy utilized, and none currently combine with RT (Table 1).
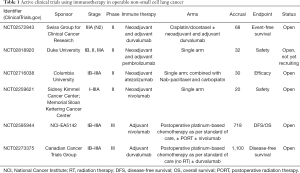
Full table
Emerging studies combining radiation and immunotherapy for effectiveness and safety
The prospect of combined modality treatment augmenting curative surgical treatment has significant advantages in NSCLC. Although approaches combining surgery, RT, chemotherapy and immunotherapy are emerging, there is already significant interest in understanding the role of adjuvant immunotherapy after definitive concurrent RT and chemotherapy. The PACIFIC trial aimed to accrue 702 patients with locally advanced NSCLC who received platinum-based chemo-RT, and then were enrolled and randomized to adjuvant durvalumab or observation if they had not progressed after initial therapy (ClinicalTrials.gov number: NCT02125461). This study is closed to accrual and results are expected sometime in 2017. RTOG Foundation 3505 study will enroll patients prior to chemo-RT and randomize 660 patients with stage III NSCLC who will receive chemo-RT followed by adjuvant nivolumab for 1 year, or observation. Both studies will analyze overall survival as their primary endpoint (ClinicalTrials.gov number: NCT02768558).
However, there are unexplored risks to combining chemo-RT, immunotherapy, and surgery. Preoperative RT may lead to lung fibrosis or lung edema, resulting in difficult surgery or concerns with wound healing (42). Postoperative RT, particularly in those who have had significant lung volume removed may have compromised lung function from pneumonitis or long-term pulmonary fibrosis.
Some potential RT toxicities may be augmented by immunotherapy, or vice versa. Grade 3 or higher pneumonitis may be seen in 2% of patients after treatment with pembrolizumab, and it is unknown the magnitude of synergy with RT and immune checkpoint modulators on pneumonitis (20). Published data on RT and immunotherapy in other disease sites suggests no significant increase in the risk of toxicity. This includes no significant increased toxicity of immunotherapy when combined with brain SRS or with pelvic RT (43-45).
Our group has shown that combining thoracic RT and immunotherapy is generally safe and yields acceptable toxicities within the range of treatment with thoracic RT alone. The most often encountered toxicities included fatigue, infection, dermatitis, and rash. Pneumonitis, primarily Grade 1 and 2, occurs in approximately 7% of patients. There were no differences in toxicity when comparing patients who received immunotherapy concurrently or sequential with RT (46).
Challenges to this approach
There is tremendous potential benefit to combining RT and immunotherapy with surgery. Given that the bulk of patients fail distantly, improving systemic relapse rates is of critical importance. However, there are significant emerging challenges in this approach.
First, we must determine which patients are most likely to benefit from this combined modality treatment by (I) identifying patients likely to fail and (II) identifying patients who will respond to RT and immunotherapy. Previous data shows that patients with high pre-SBRT SUVmax or adenocarcinoma subtype (e.g., micropapillary or solid) may be the most likely to fail local therapy, and other clinico-pathologic markers such as mutation status may enter into consideration (11,12). In addition, not all patients may respond to RT or immunotherapy. There remains discord in identifying patients who may or may not benefit from immunotherapy and what are the best methods to determine this metric (e.g., CD8 T-cells, total lymphocytes, PD-L1 expression, IL-6 plasma levels, etc.) (47). For example, studies showing patient’s with tumors expressing >50% PD-L1 may be the best responders to certain immunotherapies (20).
After patients are identified, it is unclear what treatment schema to use. There are multiple forms of immunotherapy including multiple checkpoint inhibitors with various targets, vaccines, and adoptive T-cell transfer, among others. There is no consensus in what RT total dose and fractionation to use. Finally, the timing of each of these treatments also lacks clarity. It is unclear whether how best to order therapy whether sequential or concurrent RT and immunotherapy, or if best to use these therapies in the adjuvant versus neoadjuvant setting in relation to surgery. The neoadjuvant setting allows determination of initial tumor response, and possible guidance on post-operative systemic therapy (48).
Once patients are treated with this combined modality approach, it is unclear exactly how to measure treatment response. The most obvious is clinical and imaging evidence of progression-free survival after surgery, RT, and immunotherapy. Even with routinely used imaging modalities responses to immunotherapy can present in unusual fashion such as delayed responses, pseudoprogression etc. Therefore immune-related response criteria were developed and are analyzed in many prospective studies to further evaluate the natural presentation of these immunotherapy responses (49). However, other biomarkers of treatment response should play also be included. These markers may perhaps include measures of immune response and measures of tumor response (e.g., circulating tumor DNA).
There remain a number of concerns in regards to the above with efficacy, timing, type of immunotherapy, dose and location of RT, and measuring response. However, there remains great promise in this approach combining the immune-stimulatory effects of both RT and immunotherapy to decrease systemic relapse rates in patients with otherwise curable disease.
Acknowledgements
Funding: This work is funded by MSK Cancer Center Support Grant/Core Grant (NIH P30CA008748).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-42S.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Harpole DH Jr, Herndon JE 2nd, Wolfe WG, et al. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res 1995;55:51-6. [PubMed]
- Monzó M, Rosell R, Felip E, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol 1999;17:2100-4. [Crossref] [PubMed]
- Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002;8:816-24. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Leeman JE, Rimner A, Montecalvo J, et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;97:138-45. [Crossref] [PubMed]
- Kohutek Z, Wu AJ, Zhang Z, et al. Pretreatment SUVmax on [18F]FDG-PET Is Associated With Recurrence and Survival After SBRT for Early-Stage NSCLC. Int J Radiat Oncol Biol Phys 2013;87:S12. [Crossref]
- Chi A, Liao Z, Nguyen NP, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010;94:1-11. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014; 32: abstr 8021.
- Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32: abstr 8007.
- Garon EB, Gandhi L, Rizvi N, et al. Antitumor activity of pembrolizumab (pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (PTS) with advanced non-small cell lung carcinoma (NSCLC). Annals of Oncology 2014;25:1-41. [Crossref]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [Crossref] [PubMed]
- Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol 2015;67:4-17. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Wisnivesky JP, Yankelevitz D, Henschke CI. Stage of lung cancer in relation to its size: part 2. Evidence. Chest 2005;127:1136-9. [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Zeng J, Harris TJ, Lim M, et al. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int 2013;2013:658126. [Crossref] [PubMed]
- Burnette B, Fu YX, Weichselbaum RR. The confluence of radiotherapy and immunotherapy. Front Oncol 2012;2:143. [Crossref] [PubMed]
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. [Crossref] [PubMed]
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [Crossref] [PubMed]
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015;356:82-90. [Crossref] [PubMed]
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728-34. [PubMed]
- Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One 2014;9:e101764. [Crossref] [PubMed]
- Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys 2014;88:986-97. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345-55. [Crossref] [PubMed]
- Park SS, Dong H, Liu X, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res 2015;3:610-9. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Kimura H, Matsui Y, Ishikawa A, et al. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother 2015;64:51-9. [Crossref] [PubMed]
- Zeng Y, Ruan W, He J, et al. Adoptive Immunotherapy in Postoperative Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0162630. [Crossref] [PubMed]
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5-24. [Crossref] [PubMed]
- Moraes FY, Taunk NK, Marta GN, et al. The Rationale for Targeted Therapies and Stereotactic Radiosurgery in the Treatment of Brain Metastases. Oncologist 2016;21:244-51. [Crossref] [PubMed]
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015;92:368-75. [Crossref] [PubMed]
- von Reibnitz D, Wu AJ, Barker CA Jr, et al. Safety of Combining Immune Checkpoint Inhibition and Thoracic Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;96:S156. [Crossref]
- Characiejus D, Jacobs JJ, Pašukonienė V, et al. Prediction of response in cancer immunotherapy. Anticancer Res 2011;31:639-47. [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]


