Preclinical rationale for combining radiation therapy and immunotherapy beyond checkpoint inhibitors (i.e., CART)
Introduction
In the United States, lung cancer is the second most frequently diagnosed cancer, affecting almost 225,000 people annually. It is one of the most lethal cancers resulting in over 150,000 deaths annually (1). While incremental progress has been made with combined modality treatment including surgery, radiation, and chemotherapy over the last few decades, lung cancer remains difficult to treat in its localized and distant forms with five years survival rates of 54% and 4%, respectively (2). In recent years, the introduction of several agents targeting specific oncogenic signaling pathways such as EGFR, or ALK provide additional tools to manage non-small cell lung cancer and prolong survival in a subset of patients harboring specific genomic alterations.
More recently, increasing appreciation for the role of immune surveillance in controlling aberrant cell growth has led to the development of several immunotherapy agents in various cancers including lung cancer. These agents inhibit the ability of cancer cells to evade the immune system by blocking immune checkpoint receptors such as CTLA-4 or PD-1 that normally down-regulate antitumor T cell activity upon binding to their respective ligands. Recent results in clinical trials have shown dramatic responses; for example, the phase III CheckMate 057 study of an anti-PD-1 monoclonal antibody, nivolumab, demonstrated a median survival of 12.2 months compared to 9.4 months with docetaxel in patients with advanced non-squamous non-small cell lung cancer previously treated with platinum-based doublet therapy (3). CheckMate 017 revealed a 9.2-month median overall survival in patients with squamous non-small cell lung cancer treated with nivolumab compared to 6.0 months with docetaxel when administered as second line therapy (4).
Despite the marked benefit, however, the efficacy of such agents is limited to a specific subset of patients. In CheckMate 057, while the overall objective response rate was only 19%, the response rate in patients with >1% PD-L1 expression was much higher at 38%. The median survival for this subset of PD-L1 expressing patients was also significantly higher at 17.7 months compared to 9.0 months in patients with <1% PD-L1 expression (3). The results suggest that the efficacy of these agents is limited, at least in part, due to significant heterogeneity in the expression of specific checkpoint ligands. In contrast, in Checkmate 017, there was no correlation between PD-L1 expression and response or survival even though 47% of tumor specimens in the nivolumab arm expressed PD-L1 ≥1%, (4). Furthermore, a pooled analysis of non-small cell lung cancer patients treated with nivolumab revealed that while PD-L1 expression is correlated with a greater response, patients with PD-L1 expression <1% also demonstrated improved survival when treated with nivolumab as compared to docetaxel (5). Therefore, while response and survival can correlate with PD-L1 expression, alternative markers of response are necessary to identify the subset of patient that are likely to benefit. Moreover, given that only a minority of patients benefit from nivolumab, alternative immunotherapy options are necessary in this disease, or consideration of combination immunotherapy with multiple agents and/or with radiotherapy.
A promising approach for optimizing the anti-tumor immune response that has garnered significant enthusiasm over the past few years utilizes genetically engineered T lymphocytes with tumor-specific chimeric antigen receptors (CARs). While it has been more than 25 years since Gross et al. first proposed the concept of using CAR T cell therapy to combat tumors, recent advances in receptor design, synthesis, and T cell expansion and delivery mechanisms have produced remarkable results in patients with hematological malignancies (6-12).
In this paper, we will briefly review the preclinical advances in CAR T cell therapy and clinical results in hematological malignancies, where there is the greatest available data. We will then discuss the major challenges that limit the application of CAR T cell therapy in the clinic, particularly for solid tumors. Finally, we will discuss the rationale for combining CAR T cells with radiation therapy and the potential for treatment synergy that may help overcome these challenges.
Chimeric antigen receptor modified T cells (CAR T cells)
Evading immune surveillance is one of the hallmarks of cancer (13). A major mechanism by which cancer cells trick the tumor antigen handling apparatus of the adaptive immune system is by down-regulating the expression of class I major histocompatibility complex (MHC) proteins (14). Without proper recognition of immunogenic epitopes presented on MHC molecules, cancer cells can escape cytotoxic T cell mediated responses. One approach to counteract this is by engineering T cells to express CARs that are able to recognize tumor antigens with high specificity in an MHC-independent manner. In this approach, T cells are first harvested from a patient by apheresis, purified, and then genetically engineered to express CARs specific for a cancer-associated antigen. The re-programmed T cells are then expanded ex vivo and re-infused back into the same patient.
Proper design of CARs is crucial for eliciting sustained T cell activation in a tumor specific manner. In general, CARs are constructed with two major components—an intracellular T cell signaling domain and the tumor antigen-specific extracellular domain, a single-chain variable fragment (scFv) typically derived from a monoclonal antibody—that are linked via a transmembrane domain to form a fusion chimeric molecule. In essence, a CAR combines the specificity of an antitumor antigen with the downstream T cell effector function and both the intracellular and extracellular domains have implications on the effectiveness of the CAR.
The design of the intracellular component, which promotes the effector function of a CAR, has undergone generational changes (Figure 1). First generation CARs have a singular activation domain as its intracellular signaling component, typically the cytoplasmic region of the CD3ζ or Fc receptor γ-chain derived from a T cell receptor. The in vivo efficacy of these CARs was ultimately limited due to their failure to maintain persistent T cell activation (15). This eventually led to the evolution of second generation CARs with an additional co-stimulatory domain (typically CD28, 4-1BB, or OX-40) that increases the expansion and persistence of CAR T cells (16). In third generation CARs, combinations of multiple co-stimulatory domains are added for sustained T cell activation, and while preclinical studies are promising (17-19), early phase clinical trials to test the feasibility of this approach are currently ongoing (20).
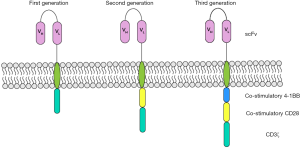
The extracellular component of a CAR is of utmost importance to provide the specificity necessary to target a tumor cell. An ideal CAR target is one that is overexpressed on cancer cells, to maximize efficacy, while not expressed on normal tissues, to minimize toxicity. These cancer-specific antigens, however, are rare. In reality, targets are chosen such that they are maximally expressed on tumor cells and minimally expressed on normal tissues to minimize the clinical implications of so-called “off-tumor, on-target” effects. In the B-cell malignancies acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma, CAR T cells directed against CD19 have demonstrated potent, durable activity (10-12,21-23). The result of a CD19-directed CAR is CD19-expressing tumor death and B-cell aplasia, an off-tumor, on-target effect that is effectively managed with intravenous immunoglobulin (11,12). As outlined below, the selection of a target is one of the challenges in the development of CAR therapy in solid tumors given lack of tumor-specific antigens with low potential for clinically significant off-tumor, on-target effects.
Challenges in adapting CAR therapy for solid malignancies
Although early results in various liquid tumors have been promising, several obstacles remain in the application of CAR T cells to solid tumors. While there are challenges to overcome in the manufacturing, expansion, and persistence of CAR T cells inherent to all CAR T cell therapies (24), other key barriers to effective use of CAR T cell therapy in solid tumors include target selection, trafficking of CARs to solid tumors, and the immunosuppressive tumor microenvironment.
Target selection
The selection of a target that maximizes anti-tumor activity with minimal side effects is the holy grail of CAR therapy in solid tumors. One such specific antigen target is epidermal growth factor variant III (EGFRvIII), an immunogenic EGFR variant found only in human tumors such as glioblastoma (25). CAR T cell therapy directed against this variant is currently under investigation (26). Most solid tumors, however, have the potential for significant off-tumor, on-target effects given their lack of specificity in protein expression. Targeting of nonspecific tumor antigens such as carboxy-anhydrase IX and ERBB2 with CAR T cell therapy have demonstrated serious off-tumor, on-target toxicities (27,28).
Potential targets of CAR therapy being evaluated in non-small cell lung cancer or all solid tumors include mesothelin (NCT02580747), MUC1 (NCT02587689, NCT02839954), GPC3 (NCT02876978), CEA (NCT02349724), HER2 (NCT02713984), and EGFR (NCT01869166)(29). Mesothelin, a differentiation antigen with no clearly defined function in normal tissue (30) that is strongly expressed in about 25% of lung adenocarcinoma, particularly in association with a KRAS-mutation (31,32), is an intriguing target; however, it is also expressed in the pericardium, pleura, peritoneum, as well as a number of different tissues throughout the body, albeit at lower levels than that seen in malignant tissues including lung adenocarcinoma (33), raising the concern for potentially dangerous adverse events. In early human clinical trials, mesothelin-directed CAR T cell therapy has been determined to be safe with no off-tumor, on-target toxicities, albeit in small patient numbers (34,35). Each potential target, however, will have potential target-specific adverse events that may limit the universal use of that CAR therapy.
CAR T cell trafficking
In contrast to hematologic malignancies where CAR T cells have exposure to circulating tumor cells bearing the desired target antigen upon infusion, CAR T cells in solid tumors have to migrate to the site of the disease. In many solid tumors, however, the cytolytic effect of the CAR T cell is limited due to restricted T cell infiltration. Trafficking of CAR T cells is controlled by similar mechanisms to normal T cells, namely T cell adhesion, tethering, chemotaxis, and extravasation (36). In solid tumors, each of these processes is dysfunctional. For example, release of angiogenic factors by tumor cells results in formation of new, albeit disorganized and leaky, blood vessels. These angiogenic factors also down-regulate adhesion molecules on the endothelial cells (37). As a result, effector T cells, including theoretically CAR T cells, are unable to efficiently migrate through the blood vessel to interact with the target (38). Moreover, the attraction of cytotoxic T cells to the tumor microenvironment is dependent on interactions between certain chemokines and their appropriate chemokine receptors, including CXCL9/CXCL10 and their receptor CXCR3 or CCL2 and its receptor CCR2. Any imbalance in the interaction between chemokine and chemokine receptor, caused by tumor cells or the associated stroma, limits trafficking of cytotoxic T cells into the tumor microenvironment (24,36). Lastly, the tumor stroma and its fibrosis is a physical barrier to T cell penetration. While non-engineered T cells generally degrade heparin sulfate proteoglycans in the extracellular matrix to penetrate this stroma, in vitro cultured T cells lack expression of the key enzyme heparanase, making it harder to penetrate the stroma (39).
Potential interventions to circumvent trafficking problems in CAR T cell therapy include anti-VEGF therapy (40), vasoactive inflammatory cytokines such as tumor necrosis factor (NGR-TNF) to upregulate adhesion molecules and decrease microenvironment hypoxia (41,42), and engineering CAR T cells with chemokine receptors to advance CAR T cell trafficking (43). Although these methods have demonstrated preclinical benefit, their clinical role in making CAR T cell therapy more effective in solid tumors remains to be validated.
Immunosuppressive tumor microenvironment
Once effector T cells are present in the tumor microenvironment, their activity in most solid tumors is hindered by immunosuppressive mechanisms. Within solid tumors, T cell activity can be inhibited by immunosuppressive mechanisms (such as PD-L1, CTLA4, and IDO), depleted amino acids (such as tryptophan and arginine), or depleted oxygen important for T cell survival, and accumulation of immunosuppressive factors such as TGF-β (24,44). Potential avenues to overcome the immunosuppressive environment include the addition of checkpoint inhibitors or VEGF inhibitors to CAR T cell therapy. Interestingly, while IDO overexpression by several tumor types has been shown to diminish the proliferation and cytotoxicity of CD19-directed CAR T cells through induction of apoptosis (45), preconditioning CAR T cell infusion with fludarabine and cyclophosphamide can inhibit IDO expression in solid tumor cell lines (46).
Radiotherapy as an adjunct to immunotherapy
In addition to the aforementioned strategies to overcome barriers to CAR T cell therapy in solid tumors, preclinical evidence supports the use of radiotherapy as an adjunct to engineered T cell therapy to potentially enhance their effectiveness. Radiotherapy, while known for direct local tumor cell death, can also elicit systemic immunomodulatory effects (47). Numerous case reports detail this abscopal effect from radiation therapy, particularly in combination with checkpoint inhibitors (48-50). In addition, several preclinical and clinical studies combining immunotherapy and radiation have elucidated the mechanism underlying the immunomodulatory effects of radiotherapy, showing response rates of the lesions outside the radiation field from 11% to 25%, and favorable overall survival (51,52).
A recent explosion in clinical trials aimed at evaluating the potential of radiotherapy in conjunction with checkpoint blockade are based on robust systemic responses observed in several preclinical tumor models (53,54). Twyman-Saint Victor et al. recently recapitulated the results of a phase 1 trial of ipilimumab and hypofractionated radiation in a melanoma mouse model. Further, they found a resistance signature predictive of response to ipilimumab and radiation therapy. The majority of cells resistant to this treatment had an upregulation of PD-L1, and the combination of radiation therapy, anti-CTLA-4, and anti-PD-L1 therapies resulted in a complete response in 80% of mice. Their results suggested that while anti-CTLA-4 therapy inhibits regulatory T cells, anti-PD-L1 therapy reverses T cell exhaustion, and radiation therapy shapes the T cell receptor repertoire of the expanded peripheral clones (52). Similarly, while the addition of an anti-α-CD137 monoclonal antibody to radiotherapy in triple-negative breast AT-3 model enhanced their response to radiation, it was noncurative until a PD-1 inhibitor was combined with radiation and anti-α-CD137 (55).
Although there is mounting evidence for the systemic immune response elicited by local radiotherapy, particularly in combination with immunomodulatory drugs, there is paucity of data directly examining the potential synergy between radiation and CAR T cell therapy. Nevertheless, a thorough examination of the preclinical data revealing the mechanisms by which radiotherapy elicits a tumor-specific immune response provides a strong rationale for using it to overcome some of the challenges faced by CAR T cell therapy in solid tumors.
Selection of a tumor-specific target for a CAR to minimize off-tumor, on-target effects is dependent on increased expression of the tumor-specific antigen on cancer cells compared to normal tissue. Radiation therapy has the potential to increase the expression of cell surface receptors and tumor-associated antigens. For example, radiation therapy is associated with a dose-dependent increase in the expression of MHC-I molecules on tumor cell surface for several days after completion of treatment (56). Importantly, radiation induces the production of novel proteins that are not present in non-irradiated cells, giving rise to new peptides for recognition by cytotoxic T cells. These results suggest that radiotherapy can enhance the immunogenicity of poorly antigenic tumors, particularly in situations where the absence of a tumor antigen is the gating factor in eliciting a tumor antigen-specific T cell response.
One specific example of how radiotherapy may affect T cell therapy examines CEA-expressing tumors, as CAR T cells directed against CEA are currently under investigation in clinical trials. Preclinical studies demonstrated that radiotherapy directed against CEA-positive M38 cells up-regulated cell surface expression of Fas, a cell surface death receptor that activates a downstream signaling cascade culminating in apoptosis upon binding its ligand FasL (57,58). Moreover, radiation sensitized CEA-positive tumors to CEA-specific T cell killing via the Fas/FasL pathway. Combination of CEA-based vaccine therapy and radiation resulted in significant cures in half of the mice treated, while neither radiation nor vaccine monotherapy achieved significant tumor control. Additionally, there was no response in tumor cells expressing dominant-negative Fas, thus supporting the role of Fas expression in facilitating an anti-tumor immune response (57,58). Similarly, a dose-dependent increase in Fas expression was noted when 23 different human colon, prostate, and lung cancer cell lines were subjected to non-lytic doses of radiation (59). Additionally, there was an increase in the expression of other surface antigens involved in T cell mediated immune responses including MUC-1, CEA, MHC-I, and intercellular adhesion molecule 1 (ICAM-1) in 21 of 23 cell lines. Moreover, five out of the five CEA-positive colon tumor cell lines exhibited significantly enhanced killing by CEA-specific T cells compared to their non-irradiated equivalents. While MUC-1 and CEA overexpression allows antigen-specific targeting of tumor cells, ICAM-1 overexpression has been shown to correlate with increased T cell adhesion and killing (60).
Another example of a target of interest in non-small cell lung cancer in which CAR T cell therapy is currently under study is mesothelin. In mesothelin-expressing xenografts in nude mice treated with a single fraction of 5 or 15 Gy radiation, the mesothelin expression per cell was found to be higher in the radiated group compared to the control group (61), consistent with prior in vitro studies (59). Subsequently, half of each group of mesothelin-expressing xenografts were treated with anti-mesothelin immunotoxin SS1(dsFv)PE38 (SS1P), which has a similar scFv as some mesothelin-directed CARs currently under investigation (35). The time to tumor doubling was substantially longer in mice treated with the combination of SS1P and radiation compared to SS1P or radiation alone or the control arm. These results show the ability of radiotherapy to enhance the efficacy of a mesothelin-specific immunotoxin by increasing the expression of its target antigen on the tumor cell surface (61). Similar increases in c-met and HER2 expression, both targets of interest in non-small cell lung cancer, are noted in the presence of radiation (62,63). While increased tumor antigens may indicate that radiotherapy to one or more malignant lesions can potentially enhance the efficacy of CAR T cell therapy through increased antigenicity of the tumor, or perhaps CAR T cell therapy could make radiation more effective, further evaluation of the combination of CARs and radiotherapy is certainly necessary to make any definitive conclusions.
Other barriers to CAR T cell therapy can also be targeted by radiotherapy. Trafficking of the CAR T cell to the tumor cells is a major issue seen with solid tumors as compared to hematologic malignancies, in part due to a decreased activated T cell adhesion, tethering, chemotaxis, and extravasation, as noted above. Radiotherapy can promote adhesion via up-regulation of adhesion molecules ICAM 1 and VCAM-1 in the tumor microenvironment in an IFN-γ dependent manner (64,65). Furthermore, chemotaxis of activated T cells into the tumor microenvironment can be enhanced by radiation. For example, ionizing radiation increases secretion of CXCL9, CXCL10, and CXCL16, chemokines important for recruitment of activated T cells (66-68). Lastly, radiation can cause remodeling of the tortuous blood vessels within the tumor, allowing for more effective delivery of cytotoxic T cells (66). In fact, remodeling the tumor environment with radiation has been shown to improve adoptive T cell transfer. In a transgenic insulinoma mouse model, the combination of radiotherapy with adoptive cell transfer of tumor-specific activated T cells prompted complete tumor regression, whereas either treatment by itself was ineffective for tumor control (66). This tumor regression was seen in the context of increased chemokine production and remodeling of the capillary network.
Once the cytotoxic T cells are within the often immunosuppressive tumor microenvironment, the role for radiation in improving cytotoxic T cell activity is less clear (69). Whereas some studies in patients undergoing chemoradiation for colon adenocarcinoma demonstrated a reduction of regulatory T cells, a similar effect was not observed in patients with breast cancer (70). Conversely, treatment of colorectal cell lines with high-dose radiation demonstrated reduced regulatory T cells and myeloid derived suppressor cells, and increased CD8+ effector T cells (71). While data is mixed, further evaluation of the effect of radiation as well as dose and fractionation on the immunosuppressive microenvironment is warranted (69,72).
Future perspective
CAR T cell therapy is a promising modality for treating solid tumors, though there are still several issues that need to be resolved to optimize its chance of success. We have outlined some potential roles that radiation may play as an adjunct to CAR T cell therapy. Unfortunately, given the early stages of development of CAR T cell therapy in solid tumors, studies combining it with radiotherapy have not yet been completed.
The preclinical effects noted in the aforementioned trials indicate the immune effects in the radiated lesion. Most CAR T cell therapy trials are done in advanced or metastatic malignancies, so it would be interesting to note whether radiotherapy has a vaccine-like effect on all metastatic lesions. Furthermore, the proper sequencing of radiation and CAR T cell therapy still needs to be determined. Should radiation therapy be given first to kill radiosensitive tumor cells and promote a more immunogenic microenvironment, in turn allowing CAR T cells to more easily penetrate the tumor and target cells with overexpressed antigens (73)?
Additionally, what is the optimal dose and fractionation of radiation? In order to answer, further efforts need to sort out mixed data presented on the effect of radiotherapy on the immunosuppressive tumor microenvironment. One study indicated that a high dose of radiation was enough to alter the immunosuppressive environment, but small doses over several fractions were ineffective. Furthermore, as there is an increase in number of clinical trials evaluating the combination of radiotherapy and checkpoint inhibitors, a trial combining radiotherapy, CAR T cell therapy, and checkpoint inhibitors to minimize the immunosuppressive environment would be interesting. This could follow from planned studies evaluating CAR T cell therapy and checkpoint inhibitors.
Particular focus will be required to carefully evaluate safety and develop ways to minimize or ameliorate serious unexpected toxicities. For example, the ROCKET trial, a phase 2 study of an anti-CD19 CAR T cell therapy JCAR015 in adults with relapsed or refractory acute lymphoblastic leukemia (NCT 02535364) was recently halted by the FDA due to multiple patient deaths (74), as a result of neurotoxicity likely secondary to fludarabine preconditioning. Given the immunomodulatory benefits of preconditioning regimens (75), one possibility that could be explored in future trials is use of radiation as a potential substitute to overcome the immunoinhibitory microenvironment.
The role of CAR T cell therapy in solid malignancies in combination with radiation therapy is unknown but remains promising. Clinical trials evaluating the feasibility of this approach are about to begin, such as a phase 1 study at Duke University (NCT02664363) which aims to evaluate the safety and efficacy of EGFRvIII CAR T cells in combination with the standard of care radiation therapy and concurrent temozolomide in newly diagnosed glioblastoma patients. Continued advancements in pairing CAR T cell therapy with other therapies such as radiation will help to advance its development.
Conclusions
CAR T cell therapy is a promising emerging tool for the treatment of solid tumors. However, minimizing normal tissue toxicity by finding appropriate tumor targets, optimizing delivery of CAR T cells to tumors, and overcoming the immunoinhibitory tumor microenvironment are some of the barriers to overcome prior to mainstream use of this promising therapy. Radiation therapy has the potential to overcome some of these challenges as it has been shown in preclinical studies to increase the expression of various tumor antigens as well as play a crucial role in chemotaxis and counteracting the inhibitory tumor microenvironment. While yet to be explored in depth, there is strong preclinical rationale for combining radiation therapy and CAR T cells in future experiments to explore the synergistic effects between these two modalities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, , based on November 2015 SEER data submission, posted to the SEER web site, April 2016.http://seer.cancer.gov/csr/1975_2013/
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Aguiar PN Jr, Santoro IL, Tadokoro H, et al. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: a network meta-analysis. Immunotherapy 2016;8:479-88. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709-20. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [Crossref] [PubMed]
- Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 2015;263:68-89. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today 1999;5:178-86. [Crossref] [PubMed]
- Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med 1995;181:1653-9. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- Tammana S, Huang X, Wong M, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther 2010;21:75-86. [Crossref] [PubMed]
- Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010;18:413-20. [Crossref] [PubMed]
- Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009;106:3360-5. [Crossref] [PubMed]
- Zhao Z, Condomines M, van der Stegen SJ, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015;28:415-28. [Crossref] [PubMed]
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Schuster SJ, Svoboda J, Nasta SD, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood 2015;126:183.
- Beatty GL, O'Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacol Ther 2016;166:30-9. [Crossref] [PubMed]
- Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res 2014;20:972-84. [Crossref] [PubMed]
- O'Rourke DM, Nasrallah M, Morrissette JJ, et al. Pilot study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. J Clin Oncol 2016;34:abstr 2067.
- Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24:e20-2. [Crossref] [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [Crossref] [PubMed]
- Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016;16:566-81. [Crossref] [PubMed]
- Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 2000;20:2902-6. [Crossref] [PubMed]
- Kachala SS, Bograd AJ, Villena-Vargas J, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res 2014;20:1020-8. [Crossref] [PubMed]
- Thomas A, Chen Y, Steinberg SM, et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget 2015;6:11694-703. [Crossref] [PubMed]
- Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 1992;50:373-81. [Crossref] [PubMed]
- Beatty GL, O'Hara MH, Nelson AM, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. J Clin Oncol 2015;33:abstr 3007.
- Tanyi JL, Haas AR, Beatty GL, et al. Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers. Cancer Res 2015;75:Abstract nr CT105.
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res 2014;74:7168-74. [Crossref] [PubMed]
- Griffioen AW, Damen CA, Martinotti S, et al. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996;56:1111-17. [PubMed]
- Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol 2013;3:231. [Crossref] [PubMed]
- Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 2015;21:524-9. [Crossref] [PubMed]
- Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010;70:6171-80. [Crossref] [PubMed]
- van Laarhoven HW, Gambarota G, Heerschap A, et al. Effects of the tumor vasculature targeting agent NGR-TNF on the tumor microenvironment in murine lymphomas. Invest New Drugs 2006;24:27-36. [Crossref] [PubMed]
- Calcinotto A, Grioni M, Jachetti E, et al. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol 2012;188:2687-94. [Crossref] [PubMed]
- Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res 2011;17:4719-30. [Crossref] [PubMed]
- Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov 2016;6:133-46. [Crossref] [PubMed]
- Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 2015;125:3905-16. [Crossref] [PubMed]
- Hanafi LA, Gauchat D, Godin-Ethier J, et al. Fludarabine downregulates indoleamine 2,3-dioxygenase in tumors via a proteasome-mediated degradation mechanism. PLoS One 2014;9:e99211. [Crossref] [PubMed]
- Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516-23. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Lock M, Muinuddin A, Kocha WI, et al. Abscopal Effects: Case Report and Emerging Opportunities. Cureus 2015;7:e344. [PubMed]
- Okuma K, Yamashita H, Niibe Y, et al. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep 2011;5:111. [Crossref] [PubMed]
- Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015;4:e1046028. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. [Crossref] [PubMed]
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728-34. [PubMed]
- Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res 2012;72:3163-74. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338-47. [Crossref] [PubMed]
- Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004;64:4328-37. [Crossref] [PubMed]
- Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004;64:7985-94. [Crossref] [PubMed]
- Zamai L, Rana R, Mazzotti G, et al. Lymphocyte binding to K562 cells: effect of target cell irradiation and correlation with ICAM-1 and LFA-3 expression. Eur J Histochem 1994;38 Suppl 1:53-60. [PubMed]
- Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res 2006;12:4983-8. [Crossref] [PubMed]
- Bhardwaj V, Zhan Y, Cortez MA, et al. C-Met inhibitor MK-8003 radiosensitizes c-Met-expressing non-small-cell lung cancer cells with radiation-induced c-Met-expression. J Thorac Oncol 2012;7:1211-7. [Crossref] [PubMed]
- Cao N, Li S, Wang Z, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res 2009;171:9-21. [Crossref] [PubMed]
- Heckmann M, Douwes K, Peter R, et al. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res 1998;238:148-54. [Crossref] [PubMed]
- Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008;180:3132-9. [Crossref] [PubMed]
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002;62:1462-70. [PubMed]
- Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008;181:3099-107. [Crossref] [PubMed]
- Siva S, MacManus M, Kron T, et al. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS One 2014;9:e109560. [Crossref] [PubMed]
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Schmidt MA, Fortsch C, Schmidt M, et al. Circulating regulatory T cells of cancer patients receiving radiochemotherapy may be useful to individualize cancer treatment. Radiother Oncol 2012;104:131-8. [Crossref] [PubMed]
- Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 2015;21:3727-39. [Crossref] [PubMed]
- Gandhi SJ, Minn AJ, Vonderheide RH, et al. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett 2015;368:185-90. [Crossref] [PubMed]
- Seyedin SN, Schoenhals JE, Lee DA, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 2015;7:967-80. [Crossref] [PubMed]
- Juno Therapeutics I. Juno Therapeutics reports clinical hold on the JCAR015 phase II ROCKET Trial. Available online: http://www.businesswire.com/news/home/20160707006375/en/Juno-Therapeutics-Reports-Clinical-Hold-JCAR015-Phase
- Turtle CJ, Hanafi LA, Berger C, et al. Addition of Fludarabine to Cyclophosphamide Lymphodepletion Improves In Vivo Expansion of CD19 Chimeric Antigen Receptor-Modified T Cells and Clinical Outcome in Adults with B Cell Acute Lymphoblastic Leukemia. Blood 2015;126:3773.


