Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation
The lung as an immune organ
Because the lungs are constantly exposed to foreign pathogens and particulates, well-established mechanisms are in place to quickly eliminate these and other types of invaders. First, the upper respiratory tract is coated with mucus that contains many antimicrobial compounds. This mucus is constantly secreted by goblet cells and expelled by ciliated epithelial cells (1). Epithelial cells that line the respiratory tract also express pattern recognition receptors (PRRs) that recognize molecular patterns associated with pathogens and other dangerous particles or cells; not only can these cells modulate the response to infection through various pathways, they also can recognize, take up, and kill pathogens (2,3). These epithelial cells also direct immune responses through cytokine recognition and secretion (2).
The next line of defense in the lung rests in immune cells. Tissue-resident alveolar macrophages are readily available for phagocytosis and for clearing debris. In fact, 95% of the airspace leukocytes are alveolar macrophages; very few are lymphocytes or neutrophils (4). Various cells in the lung, including alveolar macrophages, dendritic cells (DCs), and granulocytes also express PRRs that trigger immune responses to perturbation of homeostatic conditions. This allows quick recruitment of other immune cells to the lung that can mount an effective response and clear pathogens that may be present.
An important cell type residing in the lungs are lymphocytes. In a study of the prevalence of various lymphocyte populations in the lung, Wong et al. used cytometry by time-of-flight (CyTOF) to interrogate subsets of lymphocytes identified by surface and intracellular protein expression from healthy donors (5). CD8+ T cells and CD4+ T cells were the most prevalent subtypes in lung tissue, although natural-killer (NK) cells and NK T cells were also present. Very few B cells were found in the lungs. In vitro stimulation of lung cultures to detect intracellular cytokines revealed that the most prevalent CD4+ subset in the lung was T helper I [Th1; expresses interferon-gamma (IFN-γ)], although T helper II [Th2; interleukin (IL)-4 expressing] and regulatory T cell (Treg, IL-10 expressing) were detected at low levels. Interestingly, granulocyte macrophage colony-stimulating factor (GM-CSF) expression by the CD4+ T cells in the lung was also very high and overlapped with IFN-γ expression; production of GM-CSF by T cells has been shown to influence DC maturation (6) and drive immune disorders, specifically multiple sclerosis (7).
Immunological changes during cancer development
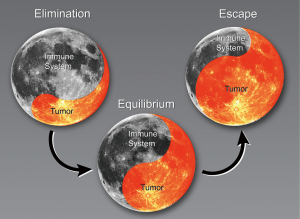
The progression of cancer with regard to the immune system has been described in three stages: elimination, equilibrium, and escape (8,9). During the elimination phase, the immune system actively attacks cancerous cells (Figure 1). The tumor reaches equilibrium with the immune system before it eventually escapes immune surveillance. At that stage, the tumors begin to grow. The following sections describe changes in the immune system that result in clinically relevant NSCLC, specifically the current understanding of immune cell populations linked to cancer and cancer outcomes derived from preclinical models, in silico sequencing analysis, and clinical evidence.
Preclinical studies of immunologic changes in lung cancer
Numerous preclinical studies have focused on the lung tumor microenvironment (TME) in efforts to reveal how tumors escape immune surveillance. In a study of the Lewis lung carcinoma (LLC) model of NSCLC, interactions of the death receptor Fas on tumor cells with its ligand did not promote apoptosis, but rather caused recruitment of myeloid-derived suppressor cells (MDSCs) to the tumor through secretion of prostaglandin E2 (PGE2) (10). PGE2 has been well studied in the context of inflammation and cancer promotion (11). That study also showed that forced overexpression of Fas on the tumor cells caused enhanced MDSC and Treg infiltration into the tumors, and depletion of MDSCs through an anti-Gr1 antibody was found to delay tumor growth. Another group also found that Fas overexpression in the LLC model led to enhanced tumor growth (12), noting that MDSC-like cells accumulated in response to cigarette smoke but that those cells did not acquire suppressive functions until after a tumor had formed (13). Drugs targeting MDSCs like gemcitabine or arginase inhibitors may facilitate T cell infiltration in the lung TME. Suzuki et al., also working with the LLC model, found that gemcitabine led to reduced numbers of MDSCs in the spleen (14), and the loss of MDSCs was reported to increase tumor growth delay in mice bearing both small and large AB12 mesothelioma tumors.
Other factors apart from PGE2 influence inflammation in preclinical lung cancer models. Kim et al. (15) found that tumor cells secreted an extracellular matrix protein called versican, which stimulated toll-like receptor (TLR) 2 on macrophages and drove the production of pro-inflammatory cytokines, specifically IL-6 and tumor necrosis factor-alpha (TNF-α). This effect was abrogated when TLR2 was knocked out in the mice; interestingly, those mice had fewer lung and liver tumors as well as prolonged. Additionally, blocking monocyte chemoattractant protein-1, also known as CCL2, can inhibit tumor growth and spontaneous metastases (16). Upon CCL2 blockade, macrophages were found to shift away from a pro-tumor phenotype, and this antitumor effect was dependent on CD8+ T cells; even though the percentages of CD8+ T cells did not increase, the expression of the activation markers CD25 and 41BB on those cells doubled.
The effect of neutrophils on the lung TME has been studied with a lung cancer model based on methylcholanthrene and hydroxytoluene exposure. In one study, depletion of Ly6G+ neutrophils led to considerable reductions in the number of tumor sites (17). However, once a tumor had formed, neutrophil depletion did not affect the growth of that tumor. These results suggest that neutrophils may be important for tumor initiation and metastasis but not tumor growth.
Overall, these studies suggest that tumors are fully capable of recruiting and polarizing myeloid cells to block adaptive immunity. They also imply that chronic inflammation may be an important factor driving the accumulation of such cells in the lung TME.
In silico methods of investigating immune-cell changes in lung cancer
Technical constraints have impeded in-depth investigations of the TME in humans over the past decade, including difficulties in identifying and studying specific types of cells in a tumor owing to intratumoral heterogeneity and identifying functional differences such as different patterns of gene expression in specific cell subsets, largely because of the inability to resolve gene expression differences at the level of individual cells or cell types (18-20). However, recent advances in single-cell genomics, transcriptomics and computational techniques have facilitated investigations of the nature of intratumoral heterogeneity in cell type and in the functional behavior of infiltrating cells (i.e., levels of PD-1 expression on the cell surface of CD4+ T cells). Much of the work in single-cell ‘omics’ has been limited to investigations of clonal diversity and tumor evolution at the level of the tumor genome, with relatively limited effort applied to evaluating non-tumor cells (19-21).
One novel way of distinguishing the individual expression profile of cell types in tumor samples is by applying a linear support vector regression-based algorithm; this approach was recently proposed and tested on existing tumor data from more than 18,000 patients from The Cancer Genome Atlas (TCGA) and the Encyclopedia of DNA Elements (ENCODE) (22,23). This method quantifies the composition of cell types by using gene expression data from tumor samples by inferring cellular compositions based on levels of expression from sets of genes based on known transcriptome profile of purified cell types.
The investigators who pioneered this algorithm evaluated tumor-infiltrating immune cell subpopulations across a range of solid tumor cell types and found that that the predominance of specific subtypes of immune cells seem to be positive predictors of outcome (23). Specifically, in lung adenocarcinoma they found that an abundance of inactivated mast cells, inactivated CD4 memory T cells, and naïve and memory B cells and plasma cells was strongly associated with favorable prognosis. On the other hand, an abundance of polymorphonuclear cells and other cells of myeloid origin was strongly associated with unfavorable prognosis. Unexpectedly, the presence of activated CD4+ memory T cells was also strongly associated with poor prognosis. In lung squamous cell carcinoma, most myeloid populations seemed to confer an unfavorable prognosis, as did memory B cells and resting CD4 memory T cells. However, the presence of activated DCs was favorable, as were activated CD4 memory, CD8, and gamma delta T cells. In both subsets of lung cancer, the presence of inactivated mast cells was associated with favorable prognosis.
Other preclinical investigations are undergoing, such as deeper phenotyping—for instance, are there specific subsets of CD4 T helper cells whose presence in tumors improve cancer outcomes? How does the memory status of helper T cells affect the TME? Does the TME differ based on patient age? Some evidence exists to suggest that inflammation increases with advanced age and is mediated through MDSCs (24). Further studies of the lung TME will probably help to answer these questions.
Clinical correlates of immune-cell subpopulations in lung cancer
Many groups have attempted to profile immune-cell subpopulations in patients with lung cancer by using techniques such as tissue microarray and immunohistochemical staining. Most such studies were done with resected tumor samples that had later been stained to evaluate immune subpopulations and their correlation with survival after surgery. Studies involving blood-based biomarkers have included samples from patients treated with a variety of modalities.
Myeloid cells
Given the prevalence of macrophages in the lung, many have investigated their role as either the tumor-suppressing (M1) or the tumor-promoting (M2) macrophage subtypes. An excellent review of tumor-associated macrophages (TAMs), their polarization, and their localization in lung cancer with regard to prognosis (25) suggested that infiltration of macrophages into tumor islets or nests (26-29) or M1 polarization (29,30) correlate with better survival in patients with lung cancer, whereas TAM density (31,32), their presence in the tumor stroma (26,27,33), and/or M2 polarization (34,35) correlate with worse survival. Other groups have found that IL-10 expression (M2-related) in TAMs (but not in tumors) at the protein level was associated with worse overall survival (36) and high IL-10 mRNA levels in TAMs were associated with tumor invasion (37). Although some studies found no correlation between TAMs and tumor progression (38-40), most have implicated TAMs in lung cancer progression.
Tumor infiltration by TAMs may not be the only relevant macrophage-related factor in lung cancer prognosis. One group showed that alveolar macrophages (obtained through bronchoalveolar lavage from patients with lung cancer) had reduced phagocytic capability (41). Incubation of alveolar macrophages with PGE2 also led to decreased phagocytosis. Another group showed that similarly obtained alveolar macrophages had increased levels of inducible nitric oxide synthase (iNOS), a traditional marker of M1 macrophage polarization (42). In addition to PGE2, chronic inflammation is also thought affect macrophage function so as to reduce phagocytic capability, perhaps because of inflammatory cytokines produced by alveolar macrophages or other myeloid cells. One group found IL-6 and IL-1 to be elevated in patients with NSCLC relative to patients with benign lung disease (43) while another showed elevated levels of TNF-α and IL-6, but not IL-1, in patients with NSCLC (44), suggesting that tumors may influence both TAMs at the tumor site and alveolar macrophages throughout the lung. Notably, IL-1, IL-6, and TNF-α tend to be associated with M1 macrophages. Could these macrophages be contributing to tumor growth, as noted in the aforementioned LLC and TLR2 preclinical study (15)? The ability of tumors to influence macrophage function may be limited spatially, as suggested by one study showing that alveolar macrophages in a non-cancerous lobe did not secrete as much TNF-α as did macrophages from a lobe containing cancer (45). Another study of bronchoalveolar lavage samples obtained well away from the affected region still demonstrated impaired phagocytosis (41). An additional study showed that macrophages from the pleural cavity and peripheral blood monocytes in patients with stage I-III lung cancer retained their phagocytic ability while producing the aforementioned pro-inflammatory cytokines (46). These studies suggest that these M1-like macrophages may not have antitumor functions, because they have lost some ability for phagocytosis and tend to create a pro- inflammatory environment. Although these cytokines can be important for antitumor effects, evidence exists to suggest that TNF-α (47,48), IL-6 (49), and IL-1 (50,51) may also participate in cancer progression.
Tumor-associated neutrophils have been less well studied with regard to lung cancer outcomes. Conflicting results have been published, with one study showing worse overall survival based on CD10 expression (52) and one showing no difference between CD10+ and CD10− tumors (53). However, in the former study, the authors compared high CD10-expressing tumors with low CD10-expressing tumors, whereas the latter study compared only CD10+ and CD10− tumors. Comparisons based on high, low, or no expression might show clearer correlations with overall survival, as suggested by studies that compared neutrophil-to-lymphocyte ratios in the blood of NSCLC patients with outcome. A meta-analysis of 14 studies comparing this ratio with outcome confirmed that a higher neutrophil-to-lymphocyte ratio correlated with worse survival in patients with lung cancer (54). Interestingly, one study found that elevated neutrophil counts alone was enough to predict poor survival (55), implying that systemic inflammation may be a risk factor in patients with cancer. Lastly, isolated neutrophils from early-stage lung tumors seem to exhibit immune-stimulatory properties; in one study, neutrophils from resected tumors that were cultured with T cells were able to drive T cell proliferation via the co-stimulatory molecules 4-1BBL, OX40L, and CD86 (56).
Elevated blood levels of MDSCs may correlate also with poorer survival. One group profiled MDSCs in blood samples of NSCLC patients and found that higher levels of MDSCs, and lower levels of DCs and monocytes, correlated with worse survival (57). Another similar study found no correlation between MDSC levels and survival, but rather that patients with NSCLC generally had increased levels of MDSC in the blood, which correlated with fewer CD8+ T cells (58), implying that the MDSCs might have more of a systemic role as compared with M2 TAMs, which seemed to be changed only locally.
The presence of tertiary lymphoid structures near tumors (59) has been investigated in patients with lung cancer. The first such study in 2008 reported the presence of these structures only in tumors and not in healthy areas of the lung among patients who had undergone surgical resection (60). These tertiary lymphoid structures were found to contain DCs, and the presence of high levels of DC-Lamp+ DCs (a maturation marker) was associated with improved disease-free survival. Notably, tumors with DC-Lamp levels also had more T cell infiltration, more B cells, and more T-bet+ Th1 (helper) cells. Tertiary lymphoid structures seem to be capable of recruiting T cells through several mechanisms (61), and their presence correlates with higher T cell numbers within both the stroma and tumor (62). DC-Lamp-high tumors have also been linked with higher 5-year overall survival rates (60% vs. 40% for DC-lamp low tumors). Another study comparing levels of B cells and DC-Lamp+ DCs in tumors reported a similar trend: tumors that had higher levels of mature DCs tended to have higher numbers of B cells, which correlated with better survival rates (63).
Lymphocytes
The importance of tumor-infiltrating lymphocytes (TILs) has been well-documented in several types of cancer. One large clinical study (956 patients) sought to correlate various immune markers with the probability of recurrence in patients with stage I adenocarcinoma (64). Higher levels of stromal Foxp3 (the transcription factor found in Tregs) and IL-7 receptor correlated with higher probability of recurrence. However, if the high Foxp3 levels were associated with high CD3 levels in the stroma, the outcomes were similar to those for low Foxp3 levels, hinting that T cell infiltration can trump Treg levels in lung cancer. The same study also showed that higher levels of IL-12 receptor beta-2 within the tumor correlated with lower probability of recurrence. A recent meta-analysis with the goal of evaluating subsets of TILs and prognosis in patients with lung cancer noted that high levels of CD3+ and CD8+ T cells correlated with improved survival, but higher stromal Foxp3 levels may have been detrimental (65).
The influence of B cells on survival is less clear. As noted previously, tertiary lymphoid structures seem to be capable of housing B cells near the tumor, and the presence of B cells has been correlated with improved survival (63), although this finding has not been consistent (66). Another study showed that infiltration of IgG4-expressing plasma cells in stromal areas correlated with high rates of survival (67), a finding that agrees with in silico results (23).
Evidence also links NK cells with improved survival. One group using CD56 as a marker for NK cells found that 88% of their evaluated patients had lower CD56 in the stroma, but the 5-year survival rate was higher among the 11% of patients with high CD56 levels (82% vs. 56%) after surgery (39). Another group found similar results with NK-cell infiltration (68).
Conclusions from the lung TME
TAMs, neutrophils, MDSCs, and Tregs all have immunosuppressive activity in the lung TME. Macrophage function seems to be altered locally, whereas MDSCs may be affected systemically. In line with other types of cancer, the presence of T cell infiltration in lung tumors, mature DCs, NK cells, and, surprisingly, B cells seems to correlate with favorable prognosis.
Radiation therapy and the immune cell response
Radiation therapy (XRT) modifies immune responses in the TME in contradictory ways. It enhances MHC class I expression, which enables the immune system to react to tumor neoantigens (69). XRT also activates immunogenic cell death via the expression of calreticulin on tumor cell surfaces and the release of ATP and HMGB1 (70,71). XRT can also promote abscopal responses with or without immunotherapy through several mechanisms, including upregulation of various trafficking receptors and CD8+ T cell recruitment (72-75), even though lymphocytes are quite sensitive to radiation and are killed shortly after XRT.
One well-studied effect of XRT is its ability to increase transforming growth factor-beta (TGF-β) levels (76). TGF-β is critical for Treg polarization and could contribute to increased Treg representation after XRT; however, Tregs are also inherently resistant to radiation (77-79). XRT also has several roles in recruiting myeloid cells to the TME (80); XRT recruits MDSCs and macrophages and, depending on the radiation dose, polarizes macrophages to the M2 phenotype. In one study of the LLC model, CD18 hypomorphic mice responded better to a single 20-Gy fraction of XRT because of reduced recruitment of myeloid cells (81). However, another group found that a higher dose (a single 30-Gy fraction) led to recruitment of mainly CD8+ T cells, and that fractionation (3 Gy ×10 fractions) led to recruitment of MDSCs (75). Indeed, these conflicting results after XRT may help to explain why some patients respond to therapy combining XRT and immunotherapies, especially immune checkpoint blockade, while others do not.
Immune checkpoint inhibitors
Our focus on the lung TME was chosen specifically to illustrate the effects of combined checkpoint inhibitors and XRT at the level of the TME. The first checkpoint inhibitor approved for NSCLC, an antibody to the programmed death 1 (PD1) receptor, inhibits a membrane protein expressed on T cells, B cells, NK cells, activated monocytes, and DCs (82). When bound by its ligand PDL1, which is expressed on the surfaces of tumor cells and myeloid cells, PD1 leads to downregulation of antigen receptor signaling, which inhibits effector cell activity (83). PD1 is also expressed by stimulated T cells, hinting that PD1+ T cells may be both tumor-specific and overstimulated (84). Both CD3+ and CD8+ T cell infiltration correlates with improved survival in NSCLC. PD1 blockade may reinvigorate these T cells, driving the immune response seen in the clinic. Tumors with high PDL1 expression are known to have decreased numbers of TILs (85) and to respond better to anti-PD1 monoclonal antibody than do tumors with low PDL1 levels, suggesting that even if T cells do express PD1, they may not be signaling through PD1 unless the tumor is actively expressing PDL1 (86).
The second common checkpoint inhibitor, anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4), is targeted to a receptor expressed on T cells, extensively so on Tregs. When CTLA4 is bound by CD80 or CD86 on an antigen-presenting cell, it transmits an inhibitory signal to T cells but promotes Treg immunosuppressive functions (87). Because CTLA4 binds to CD80 and CD86 with greater affinity than to the costimulatory receptor CD28, T cell activation leads to increased expression of CTLA4 as a means of negative feedback to prevent autoimmune reactions (88). Recent evidence suggests that the primary mode of action of anti-CTLA4 drugs in mice is not to promote stimulation of effector T cells but to deplete Tregs via antibody-dependent cell-mediated cytotoxicity (89).
Although PD1 targets T cells that have already been activated and CTLA4 may deplete Tregs, it is important to note that other immune cells are present in the lung TME; myeloid cells in particular seem to have large role in immunosuppression.
Preclinical studies of PD1 or CTLA4 + XRT
Few preclinical studies have been done that combine anti-PD1 or anti-CTLA4 and XRT in models of lung cancer. In one such study, combining PD1 blockade with XRT in a mouse model of KRAS-mutant NSCLC yielded significantly improved survival and smaller tumor volumes in comparison with control and monotherapy groups (90). XRT + anti-PD1 was capable of causing regression in the 344SQ (p53 and KRAS mutant) NSCLC line (91), and two of eight mice treated with this modality experienced complete tumor regression and were resistant to tumor rechallenge. The anti-CTLA4 antibody was first reported to increase the antitumor activity of XRT in 2014 (92); combining anti-CD25 with XRT in a lung cancer model led to significant decreases in Tregs both at the irradiated site and distally at nonirradiated sites relative to either therapy given alone (93). One could expect similar results with anti-CTLA4 and XRT for the following reasons: (I) CTLA4 acts to reduce Tregs; (II) Tregs seem to have important roles in the lung TME; (III) and XRT may increase the numbers of Tregs in the lung TME.
Future directions
Applying XRT to multiple sites of disease
Key questions remaining for the use of XRT with immunotherapy are the optimal radiation dose and schedule, tumor location, and extent of tumor to be irradiated. Several of the ongoing trials of XRT plus immunotherapy treat only one metastatic site; however, targeting multiple sites or even all areas of gross disease may be much more likely to improve systemic responses. One advantage of such an approach would be to prompt the release of greater numbers of neoantigens from different sites of metastasis, which would improve the probability of priming more T cells, perhaps translating into improved systemic control. Another challenge to the destruction of cancer via the immune system is low T cell penetration into the tumor; for example “cold” tumors without T cells are much less likely to respond to checkpoint inhibitors. Destroying all sites of gross disease with XRT would abrogate this problem. Irradiation of all disease sites may not be feasible for some patients, and it could well increase toxicity, especially for colitis from treating abdominal disease. Recent trials have demonstrated improvements in outcome from aggressive ablative therapy for patients with up to 3 sites of metastatic NSCLC (94), and there could be further improvement with the addition of immunotherapy.
Using XRT to increase response rates to adoptive T cell therapy
Adoptive T cell therapy has recently gained attention for its potential as systemic antitumor therapy. Because TILs consist of CD4+ and CD8+ lymphocytes, some TILs directly destroy tumors and others promote stimulation of other immune cells (B cells, macrophages, CD8+ T cells) to promote tumor-cell lysis (95). This may explain why the presence of TILs is a prognostic factor for overall and event-free survival as well as recurrence in melanoma, breast cancer, and ovarian cancer (96-98). Efforts have begun to investigate the infusion of expanded autologous T cells isolated from resected tumors.
Given the ability to isolate, extract, and expand TILs, XRT has taken on new promise for improving distant disease control. Metastatic disease is common in NSCLC, for which the mainstay treatment is chemotherapy. However, chemotherapy is lymphocyte-depleting and (99) cannot penetrate the blood-brain barrier. Replacing chemotherapy with tumor-specific T cells may be a way of resolving this difficult issue, as preclinical studies have shown that T cells can penetrate the blood-brain barrier (100,101). Infusing TILs that have been expanded ex vivo with the greater antigen receptor diversity generated from definitive XRT could enhance the penetration of those cells into metastases in the brain and at other sites.
Conclusions
Immunotherapy has profoundly changed the care of lung cancer patients. Maximizing its utility requires a deep understanding of the TME. Although anti-PD1 agents have shown to be effective against NSCLC, additional immune cell populations will need to be targeted to increase response rates. Of all the immune cell populations that have been implicated in NSCLC, immunotherapies targeting TAMs and MDSCs are likely the most critical because XRT recruits these cells to the TME. XRT could also be used to recruit T cells to multiple sites and, with immunotherapy, could also be useful for enhancing the production of TILs, which could then be harvested for expansion as a more diverse population of tumor-specific T cells. These and other strategies will lead to improved clinical outcomes for patients with NSCLC.
Acknowledgements
The authors are indebted to Christine F. Wogan of MD Anderson’s Division of Radiation Oncology for editorial assistance and David M. Aten in MD Anderson’s Medical Illustration and Multimedia for graphics assistance.
Funding: This work was supported by an MD Anderson Knowledge Gap award, Doctors Cancer Foundation Grant, The Lung Cancer Research Foundation, Cancer Center Support (Core) Grant CA016672 from the NCI (to The University of Texas MD Anderson Cancer Center), the Mabuchi Research Fund, the family of M. Adnan Hamed, the Susan and Peter Goodwin Foundation, the Orr family, and the VanSteklenburg family.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev 2000;173:27-38. [Crossref] [PubMed]
- Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol 2008;40:1348-61. [Crossref] [PubMed]
- Kowalski MP, Dubouix-Bourandy A, et al. Host resistance to lung infection mediated by major vault protein in epithelial cells. Science 2007;317:130-2. [Crossref] [PubMed]
- Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc 2005;2:403-11. [Crossref] [PubMed]
- Wong MT, Ong DE, Lim FS, et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016;45:442-56. [Crossref] [PubMed]
- Min L, Mohammad Isa SA, Shuai W, et al. Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol 2010;184:4625-9. [Crossref] [PubMed]
- Rasouli J, Ciric B, Imitola J, et al. Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-beta Therapy. J Immunol 2015;194:5085-93. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Zhang Y, Liu Q, Zhang M, et al. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol 2009;182:3801-8. [Crossref] [PubMed]
- Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 2013;35:123-37. [Crossref] [PubMed]
- Lee JK, Sayers TJ, Back TC, et al. Lack of FasL-mediated killing leads to in vivo tumor promotion in mouse Lewis lung cancer. Apoptosis 2003;8:151-60. [Crossref] [PubMed]
- Ortiz ML, Lu L, Ramachandran I, et al. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res 2014;2:50-8. [Crossref] [PubMed]
- Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. [Crossref] [PubMed]
- Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009;457:102-6. [Crossref] [PubMed]
- Fridlender ZG, Kapoor V, Buchlis G, et al. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol 2011;44:230-7. [Crossref] [PubMed]
- Vikis HG, Gelman AE, Franklin A, et al. Neutrophils are required for 3-methylcholanthrene-initiated, butylated hydroxytoluene-promoted lung carcinogenesis. Mol Carcinog 2012;51:993-1002. [Crossref] [PubMed]
- Navin NE. The first five years of single-cell cancer genomics and beyond. Genome Res 2015;25:1499-507. [Crossref] [PubMed]
- Yu P, Lin W. Single-cell Transcriptome Study as Big Data. Genomics Proteomics Bioinformatics 2016;14:21-30. [Crossref] [PubMed]
- de Bourcy CF, De Vlaminck I, Kanbar JN, et al. A quantitative comparison of single-cell whole genome amplification methods. PloS One 2014;9:e105585. [Crossref] [PubMed]
- Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. [Crossref] [PubMed]
- Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453-7. [Crossref] [PubMed]
- Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. [Crossref] [PubMed]
- Bowdish DM. Myeloid-derived suppressor cells, age and cancer. Oncoimmunology 2013;2:e24754. [Crossref] [PubMed]
- Conway EM, Pikor LA, Kung SH, et al. Macrophages, Inflammation, and Lung Cancer. Am J Respir Crit Care Med 2016;193:116-30. [Crossref] [PubMed]
- Welsh TJ, Green RH, Richardson D, et al. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 2005;23:8959-67. [Crossref] [PubMed]
- Dai F, Liu L, Che G, et al. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer 2010;10:220. [Crossref] [PubMed]
- Kim DW, Min HS, Lee KH, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer 2008;98:1118-24. [Crossref] [PubMed]
- Ohri CM, Shikotra A, Green RH, et al. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 2009;33:118-26. [Crossref] [PubMed]
- Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010;10:112. [Crossref] [PubMed]
- Chen JJ, Yao PL, Yuan A, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res 2003;9:729-37. [PubMed]
- Arenberg DA, Keane MP, DiGiovine B, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother 2000;49:63-70. [Crossref] [PubMed]
- Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008;113:1387-95. [Crossref] [PubMed]
- Zhang B, Yao G, Zhang Y, et al. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo) 2011;66:1879-86. [Crossref] [PubMed]
- Ohtaki Y, Ishii G, Nagai K, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol 2010;5:1507-15. [Crossref] [PubMed]
- Zeni E, Mazzetti L, Miotto D, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J 2007;30:627-32. [Crossref] [PubMed]
- Wang R, Lu M, Zhang J, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res 2011;30:62. [Crossref] [PubMed]
- Toomey D, Smyth G, Condron C, et al. Infiltrating immune cells, but not tumour cells, express FasL in non-small cell lung cancer: No association with prognosis identified in 3-year follow-up. Int J Cancer 2003;103:408-12. [Crossref] [PubMed]
- Al-Shibli K, Al-Saad S, Donnem T, et al. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology 2009;55:301-12. [Crossref] [PubMed]
- Carus A, Ladekarl M, Hager H, et al. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer 2013;81:130-7. [Crossref] [PubMed]
- Dehle FC, Mukaro VR, Jurisevic C, et al. Defective lung macrophage function in lung cancer +/- chronic obstructive pulmonary disease (COPD/emphysema)-mediated by cancer cell production of PGE2? PloS One 2013;8:e61573. [Crossref] [PubMed]
- Liu CY, Wang CH, Chen TC, et al. Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. Br J Cancer 1998;78:534-41. [Crossref] [PubMed]
- Matanić D, Beg-Zec Z, Stojanović D, et al. Cytokines in patients with lung cancer. Scand J Immunol 2003;57:173-8. [Crossref] [PubMed]
- Chyczewska E, Mroz RM, Kowal E. TNF-alpha, IL-1 and IL-6 concentration in bronchoalveolar lavage fluid (BALF) of non-small cell lung cancer (NSCLC). Rocz Akad Med Bialymst 1997;42 Suppl 1:123-35. [PubMed]
- Siziopikou KP, Harris JE, Casey L, et al. Impaired tumoricidal function of alveolar macrophages from patients with non-small cell lung cancer. Cancer 1991;68:1035-44. [Crossref] [PubMed]
- Kimura S, Sone S, Takahashi K, et al. Antitumour potential of pleural cavity macrophages in lung cancer patients without malignant effusion. Br J Cancer 1989;59:535-9. [Crossref] [PubMed]
- Lebrec H, Ponce R, Preston BD, et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin 2015;31:557-74. [Crossref] [PubMed]
- Yan HQ, Zhang D, Shi YY, et al. Ataxia-telangiectasia mutated activation mediates tumor necrosis factor-alpha induced MMP-13 up-regulation and metastasis in lung cancer cells. Oncotarget 2016;7:62070-83. [PubMed]
- Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014;141:125-39. [Crossref] [PubMed]
- Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 2006;25:387-408. [Crossref] [PubMed]
- Dmitrieva OS, Shilovskiy IP, Khaitov MR, et al. Interleukins 1 and 6 as Main Mediators of Inflammation and Cancer. Biochemistry (Mosc) 2016;81:80-90. [Crossref] [PubMed]
- Kadota K, Nitadori J, Ujiie H, et al. Prognostic Impact of Immune Microenvironment in Lung Squamous Cell Carcinoma: Tumor-Infiltrating CD10+ Neutrophil/CD20+ Lymphocyte Ratio as an Independent Prognostic Factor. J Thorac Oncol 2015;10:1301-10. [Crossref] [PubMed]
- Kristiansen G, Schluns K, Yongwei Y, et al. CD10 expression in non-small cell lung cancer. Anal Cell Pathol 2002;24:41-6. [Crossref] [PubMed]
- Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524-30. [Crossref] [PubMed]
- Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950-8. [Crossref] [PubMed]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466-80. [Crossref] [PubMed]
- Vetsika EK, Koinis F, Gioulbasani M, et al. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res 2014;2014:659294. [Crossref] [PubMed]
- Liu CY, Wang YM, Wang CL, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136:35-45. [Crossref] [PubMed]
- Dieu-Nosjean MC, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014;35:571-80. [Crossref] [PubMed]
- Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26:4410-7. [Crossref] [PubMed]
- de Chaisemartin L, Goc J, Damotte D, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011;71:6391-9. [Crossref] [PubMed]
- Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705-15. [Crossref] [PubMed]
- Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014;189:832-44. [Crossref] [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013;31:490-8. [Crossref] [PubMed]
- Geng Y, Shao Y, He W, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem 2015;37:1560-71. [Crossref] [PubMed]
- Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol 2010;185:4977-82. [Crossref] [PubMed]
- Fujimoto M, Yoshizawa A, Sumiyoshi S, et al. Stromal plasma cells expressing immunoglobulin G4 subclass in non-small cell lung cancer. Hum Pathol 2013;44:1569-76. [Crossref] [PubMed]
- Jin S, Deng Y, Hao JW, et al. NK cell phenotypic modulation in lung cancer environment. PloS One 2014;9:e109976. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014;3:e28518. [Crossref] [PubMed]
- Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front Immunol 2013;4:68. [Crossref] [PubMed]
- Brooks ED, Schoenhals JE, Tang C, et al. Stereotactic Ablative Radiation Therapy Combined With Immunotherapy for Solid Tumors. Cancer J 2016;22:257-66. [Crossref] [PubMed]
- Schoenhals JE, Seyedin SN, Tang C, et al. Preclinical Rationale and Clinical Considerations for Radiotherapy Plus Immunotherapy: Going Beyond Local Control. Cancer J 2016;22:130-7. [Crossref] [PubMed]
- Seyedin SN, Schoenhals JE, Lee DA, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 2015;7:967-80. [Crossref] [PubMed]
- Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015;21:3727-39. [Crossref] [PubMed]
- Dancea HC, Shareef MM, Ahmed MM. Role of Radiation-induced TGF-beta Signaling in Cancer Therapy. Mol Cell Pharmacol 2009;1:44-56. [Crossref] [PubMed]
- Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys 2011;81:1128-35. [Crossref] [PubMed]
- Persa E, Balogh A, Sáfrány G, et al. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett 2015;368:252-61. [Crossref] [PubMed]
- Balogh A, Persa E, Bogdandi EN, et al. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm Res 2013;62:201-12. [Crossref] [PubMed]
- Vatner RE, Formenti SC. Myeloid-derived cells in tumors: effects of radiation. Semin Radiat Oncol 2015;25:18-27. [Crossref] [PubMed]
- Ahn GO, Tseng D, Liao CH, et al. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A 2010;107:8363-8. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [Crossref] [PubMed]
- Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014;124:2246-59. [Crossref] [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Perkins D, Wang Z, Donovan C, et al. Regulation of CTLA-4 expression during T cell activation. J Immunol 1996;156:4154-9. [PubMed]
- Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32-42. [Crossref] [PubMed]
- Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016;1:e87415. [Crossref] [PubMed]
- Wang X, Schoenhals JE, Li A, et al. Suppression of Type I IFN Signaling in Tumors Mediates Resistance to Anti-PD-1 Treatment That Can Be Overcome by Radiotherapy. Cancer Res 2017;77:839-50. [Crossref] [PubMed]
- Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PloS One 2014;9:e92572. [Crossref] [PubMed]
- Son CH, Bae JH, Shin DY, et al. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys 2015;92:390-8. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Radvanyi LG. Tumor-Infiltrating Lymphocyte Therapy: Addressing Prevailing Questions. Cancer J 2015;21:450-64. [Crossref] [PubMed]
- Salgado R, Denkert C, Campbell C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol 2015;1:448-54. [Crossref] [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [Crossref] [PubMed]
- Schatton T, Scolyer RA, Thompson JF, et al. Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol 2014;1102:287-324. [Crossref] [PubMed]
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084-91. [Crossref] [PubMed]
- Yu JS, Lee PK, Ehtesham M, et al. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol 2003;64:55-61. [Crossref] [PubMed]
- Lu W, Su J, Kim LS, et al. Active specific immunotherapy against occult brain metastasis. Cancer Res 2003;63:1345-50. [PubMed]


