Novel systemic therapy against malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) arises from the normal mesothelioma cells of the pleura and is generally associated with a poor prognosis, as many patients are diagnosed with advanced disease, and long-term disease free survival is rare even in the early stage setting. Physicians in the United States diagnose approximately 2,500 new cases per year (1). MPM affects more men than women with an incidence ratio of 3.8:1, and men have a lower overall 5-year survival (4.5%) compared to women (13.4%) (2). The median age of diagnosis in the United States is 72 years. Asbestos exposure was first identified as a cause of MPM in the 1960’s, and it is estimated that up to 80% of cases of MPM are related to asbestos exposure with the onset of disease generally occurring 20–70 years after exposure through mechanisms of chronic inflammation (3-5). The incidence of MPM in the United States has stabilized over the past few years likely due to decreased asbestos use since the 1970’s (1); however, the incidence of MPM in developing countries is expected to increase and represents a substantial health and economic burden (6,7). Risk factors for mesothelioma also include environmental, occupational, and para-occupational exposure to asbestos and other mineral fibers such as erionite (8). Prior chest radiation therapy or occupational radiation also increases the risk for developing mesothelioma (9-15). Familial variants of mesothelioma exist as well: for example, two families with strong family history of mesothelioma without an associated history of exposure to asbestos or other mineral fibers were found to have familial mutations in BRCA associated protein 1 (BAP1), a tumor suppressor gene, that either affects the gene’s promoter or forms a premature stop codon (16). Somatic mutations of BAP-1 have also been identified in 57–63% of cases (17).
Approximately 60% of patients with MPM present with pleural effusion, dyspnea, and chest wall pain (18). The disease is typically locally invasive or even more extensive at presentation. The diagnosis of MPM requires adequate tissue in the context of appropriate clinical, radiographic, and surgical findings. Thoracoscopic biopsy is considered the gold standard diagnostic method and case series have reported diagnostic sensitivity to range from 94–98% (19-21). CT-guided needle biopsy is also commonly used and has a reported sensitivity of 83–88% (22-26). The International Mesothelioma Interest Group (IMIG) established diagnostic criteria based on cytology. These have low sensitivity ranging from 32–76%, because of the challenges in distinguishing a collection of benign mesothelial cells from invasive mesothelioma, but a high positive predictive value approaching 100% (27). Markers used to distinguish MPM from other types of pleural masses include cytokeratin 5/7, Wilm’s Tumor 1 (WT1), D2-40 (podoplanin), and calretinin, Pathologists classify MPM tumors into three histological subtypes: epithelioid, sarcomatoid (which includes desmoplastic and lymphohistiocytic variants), or biphasic. The epithelioid histology occurs in 50–60% of patients and has a more favorable prognosis. The sarcomatoid histology comprises approximately 20% of cases and is associated a less favorable prognosis, as well as a lower chance of response to therapy.
Once the diagnosis is made, patients are staged based on the IMIG TNM staging system. All patients undergo CT imaging of the chest and abdomen, and ideally PET/CT scan is performed in patients being considered for surgery to evaluate for extrathoracic spread. Surgeons will often use MRI of the chest and/or abdomen for further evaluation when there is suspected diaphragmatic, spine, or vascular invasion. Additional procedures can be performed to exclude extra-pleural disease, including VATS to evaluate the contralateral pleura, and laparoscopy to rule out peritoneal spread prior to resection.
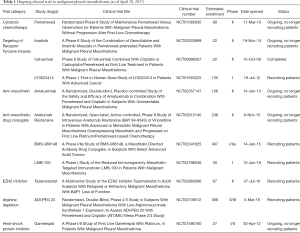
Management options presented to patients with MPM are determined based on the age and functional status of the patient and on the stage (including lymph node involvement), histology, and resectability of the disease. Younger patients with good performance status, epithelioid histology, and localized resectable disease can generally be offered multimodality therapy with systemic chemotherapy, surgical resection, and sometimes radiation therapy (28,29). Another article in this issue of Translational Lung Cancer Research discusses surgical options for MPM; however, we will briefly mention here that surgical options are generally limited to patients with the epithelioid subtype of MPM and consist of pleurectomy/decortication (P/D) with mediastinal lymph node sampling or extrapleural pneumonectomy (EPP). Patients who will not benefit from P/D or EPP can be offered palliative systemic chemotherapy, depending on their functional status. While palliative radiation therapy can help improve symptoms from invasive disease, definitive radiation therapy has not been shown to be effective after an incomplete surgical resection and has elevated toxicity to the intact lung (30). While aggressive therapy is more effective in patients with early, limited MPM with epithelioid history (31,32), most patients present with higher stage disease or cannot tolerate extensive surgical resection due to advanced age and/or medical co-morbidities. These patients should be considered for systemic therapy. Even with treatment, MPM has a poor prognosis with median survival of approximately one-year and cure is very rare (33-35). The limited efficacy of therapy for MPM highlights the need to develop more effective therapies for MPM which is challenged by the heterogeneity of MPM (with three pathological subtypes), the relatively low incidence of the disease, and the degree of difficulty with assessment of response. Here, we review recent efforts to improve systemic therapies for MPM with ongoing trials listed in Tables 1 and 2.
Full table

Full table
Cytotoxic chemotherapy for MPM
Surgical resection alone does not generally mitigate microscopic localized or metastatic disease, so adjuvant chemotherapy has been evaluated and shown to provide added benefit (28). Patients with medically inoperable mesothelioma are managed with observation, best supportive care, or systemic chemotherapy. Anti-metabolites such as pemetrexed, raltitrexed, and methotrexate, platinum analogs (cisplatin and carboplatin), gemcitabine, vinorelbine, and doxorubicin have activity in MPM with single-agent response rates of 7–20% (36). Anti-folate therapy with pemetrexed, combined with platinum therapy, with or without bevacizumab, is the current standard first-line systemic therapy for advanced or unresectable MPM (37,38).
The history leading to establishing anti-folate, platinum, and bevacizumab as front-line therapy spans decades. In the late 1980’s and 1990’s, randomized trials evaluated the efficacy of multiple single-agent chemotherapeutics including anthracyclines, topoisomerase inhibitors, taxanes, alkylating agents, and platinum analogues in MPM with low response rates of 0–13%, progression free survival of 2–5 months, and median overall survival ranging 5–8 months (39-43). Overall, single agent chemotherapy was persistently been found to have a modest response in patients with mesothelioma, with slightly better responses of around 23% (95% CI: 19.7–26.8) observed with cisplatin across many clinical trials (36,44).
Combination of pemetrexed added to platinum therapy
In 2003, Vogelzang et al. published the landmark phase III EMPHACIS trial reporting that addition of pemetrexed to cisplatin improved outcomes for patients with MPM (37). Chemotherapy naïve patients who were not eligible for curative surgery were randomized to 500 mg/m2 pemetrexed and 75 mg/m2 of cisplatin (n=226) or cisplatin alone (n=222) every 21 days. The addition of pemetrexed to cisplatin improved the response rate from 16.7–41.3% compared to cisplatin alone. The median time to progression was significantly longer in the pemetrexed plus cisplatin group at 5.7 months compared to 3.9 months in the cisplatin alone group (P=0.001). The median overall survival in the pemetrexed group was 12.1 months compared to 9.3 months in the cisplatin alone group (P=0.02). Similarly, van Meerbeeck et al. conducted a European randomized phase III clinical trial (EORTC) with 250 patients randomized to cisplatin 80 mg/m2 either alone or with raltitrexed 3 mg/m2 (45). The response rate was 13.6% in the cisplatin alone arm and 23.6% in the cisplatin plus raltitrexed arm (P=0.056). Similar to the EMPHACIS trial (37), the addition of this anti-folate therapy to cisplatin improved median overall survival by three months from 8.8 months (95% CI: 7.8–10.8) to 11.4 months (95% CI: 10.1–15.0).
In clinical practice, carboplatin is often substituted for cisplatin due to its reduced risk of toxicity (46). Phase II data demonstrated the efficacy of pemetrexed (500 mg/m2) plus carboplatin AUC 5 in MPM (47-49). Santoro et al. reported a slightly lower response rate of 21.7% (95% CI: 18.8–24.8) for carboplatin-based chemotherapy compared to a response rate of 26.3% (95% CI: 23.2–29.6) for cisplatin-based chemotherapy when combined with pemetrexed. However, this study observed that time to progression and 12-month survival were essentially equivalent with both regimens (50).
Gemcitabine with platinum therapy
Gemcitabine appears to be an active drug in MPM as well. A retrospective series of 81 MPM patients treated first-line with a platinum analog plus gemcitabine (n=40) or pemetrexed (n=41) showed that the efficacy of gemcitabine and pemetrexed platinum doublets are similar (51). Byrne et al. observed partial responses in 10 out of 21 (47.5%, 95% CI: 26.2–69) patients with MPM treated with cisplatin 100 mg/m2 on day 1 and gemcitabine 1,000 mg/m2 on days 1, 8, 15 of a 28-day cycle for six cycles (52). This same regimen was further evaluated in a multicenter phase II study with 52 patients with MPM of which 17 (33%, 95% CI: 20–46) had a partial response (53). Kalmadi et al. reported a 12% response rate (95% CI: 5–24%) with cisplatin divided into weekly doses at 30 mg/m2 to reduce toxicity (54). Ak et al. found no difference in survival between patients who received platinum therapy with pemetrexed compared to those who received platinum therapy with gemcitabine (55). Carboplatin with gemcitabine has also been tested in MPM with a response rate of 26% (95% CI: 15–40%) with acceptable toxicity (56). Overall, gemcitabine combined with platinum agents appears to be an active regimen in MPM; however, there is heterogeneity between trials with response rates ranging 12–48% and median survival ranging from 9.5 to 12 months (52-54,57,58).
Vinorelbine
Single agent therapy with the semisynthetic vinca alkaloid vinorelbine has a response rate of 24% with low toxicity (59). In the front line setting, vinorelbine combined with oxaliplatin has a 23% response rate (95% CI: 9–44%) (60). In the relapsed setting, patients who have had a prior chemotherapy demonstrated a 16% response rate to vinorelbine 30 mg/m2 for 6 weeks (61). A phase III trial randomized 409 patients to receive active symptom control with or without chemotherapy which consisted of either four cycles of mitomycin C 6 mg/m2, vinblastine 6 mg/m2, and cisplatin 50 mg/m2 every 21 days, or 12 weekly doses of vinorelbine 30 mg/m2 (62). Overall, there was a trend towards improvement in survival with vinorelbine; however, the study was underpowered due to poor accrual in the setting of platinum and pemetrexed becoming preferred first-line therapy for MPM (37,62-64).
2nd line and salvage therapy
Generally, patients of good performance status who relapse after frontline therapy can be considered for retreatment with the initial regimen depending on the interval of disease control, or second line therapy with gemcitabine or vinorelbine based on the evidence discussed above. Patients in the EMPHACIS trial who received post-study chemotherapy had a statistically prolonged survival with an adjusted hazard ratio of 0.56 (CI: 0.44–0.72), demonstrating benefit of additional systemic therapy after first line treatment (65). Prior to cisplatin and pemetrexed becoming the first-line standard of care, Jassem et al. showed that pemetrexed increased progression free survival in previously treated, pemetrexed-naïve patients (66). Patients with relapsed disease after pemetrexed-based first-line therapy can be considered for retreatment with pemetrexed based chemotherapy if disease control is achieved for more than 12 months after administration of first line therapy (67). There are ongoing investigations regarding the role of maintenance pemetrexed therapy, because this approach has a superior overall survival in patients with non-small cell lung cancer (68,69). A case report demonstrated that pemetrexed maintenance therapy is feasible in MPM (70), and a clinical trial investigating the efficacy of pemetrexed maintenance is currently ongoing (clinicaltrials.gov NCT01085630).
Anti-angiogenic therapy
Tumor growth requires angiogenesis, the growth of new blood vessels into a tumor to supply oxygen and other nutrients (71). Several anti-angiogenesis strategies have been tested clinically in MPM. Thalidomide was first shown to inhibit angiogenesis in a corneal micro-pocket assay (72). Based on these, and other findings (73), clinical investigators have tested thalidomide in patients with MPM. The randomized phase III NVALT 5 trial compared thalidomide maintenance therapy to best supportive care after at least four cycles of pemetrexed with platinum therapy. Unfortunately, no benefit was noted in time to progression (3.6 months in the thalidomide group versus 3.5 months in the best supportive care group) with the addition of thalidomide maintenance to first-line chemotherapy (74).
The Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS) was initiated as a phase II trial to determine if adding bevacizumab, a monoclonal antibody against the endothelial cell mitogen Vascular Endothelial Growth Factor-A (VEGF-A), to cisplatin and pemetrexed provided clinical benefit. This study met the primary outcome with 27 patients out of 47 patients achieving disease control at 6 months without unexpected toxicity signals. The study then expanded to a randomized, controlled, open-label, phase III trial with 448 patients. The addition of bevacizumab to cisplatin and pemetrexed significantly increased overall survival to 18.8 months in patients who received bevacizumab, cisplatin, and pemetrexed (n=223) vs. 16.1 months in patients who received cisplatin and pemetrexed (n=225) (38). Based on this trial, consideration should be given to bevacizumab in front-line therapy with pemetrexed and cisplatin or carboplatin in clinical practice.
Clinical trials have also investigated the efficacy of small molecule inhibitors of angiogenesis. Nintedanib is an intracellular inhibitor of tyrosine kinase receptor signaling with specificity for VEGR1-3, platelet derived growth factor receptor (PDGFR)-α and -β, and fibroblast growth factor receptor (FGFR)1–3. Grosso et al. presented positive data at the 17th IASLC World Conference on Lung Cancer reporting the addition of nintedanib versus placebo to chemotherapy with cisplatin and pemetrexed increased progression free survival (9.4 vs. 5.7 months, P=0.0174). There was also a preliminary trend towards improved overall survival (18.3 vs. 14.5 months, P=0.4132) but further investigation is needed to statistically confirm this survival benefit (75). Cediranib is a potent small molecule inhibitor of VEGFR1-3, c-Kit, and PDGFR-β signaling (76,77). Patients with MPM who had previously been treated with platinum containing therapy demonstrated a 9–10% response rate to therapy with cediranib 45 mg po daily (78,79). Tsao et al. reported the phase I portion of the SWOG 0905 evaluating cediranib combined with standard of care platinum and pemetrexed therapy followed by cediranib maintenance therapy in chemotherapy naive patients with unresectable MPM (80). Patients who received 6 cycles of cediranib in combination with platinum and pemetrexed therapy followed by cediranib maintenance therapy (20 mg daily) had a median PFS of 13 months and OS of 16 months, which is better than expected from historical controls (37,80).
Sunitinib, which targets VEGFR1-3, demonstrated a response in only one treatment naïve patient out of 35 total enrolled, in a study including MPM patients with and without prior therapy (81). Similarly, vatalanib demonstrated a low response rate in a phase II study (82). Sorafenib, a potent inhibitor of the RAS/MEK pathway which also targeted VEGFR and cKIT, had a response rate of 6% at a dose of 400 mg po BID in patients with unrespectable MPM with or without prior therapy (83). Another phase II trial reported that 36% patients treated with sorafenib 400 mg po BID were progression free at 6 months (84). While these findings are comparable to other small molecular inhibitors of angiogenesis, additional studies are needed to determine if this translates into clinical benefit. As discussed below, pre-clinical studies demonstrate efficacy of sorafenib combined with everolimus in mesothelioma xenografts (85), which provides a rationale for combination studies with multiple agents.
PDGFR inhibitors
Normal mesothelial cells express PDGFR-α while mesothelioma tumors express both PDGF-AA and PDGF-BB ligands as well as PDGFR-α and PDGFR-β (86-88). While the staining pattern of PDGFR-β immunoreactivity in frozen sections of MPM are consistent with MPM tumor cell expression of PDGFR-β, the function of PDGFR-β within tumor cells, as opposed to the associated stroma, has not been well described. The finding of high PDGF-AA and PDGF-BB ligands and PDGFR-α and PDGFR-β expression in MPM tumors led to the hypothesis that targeting PDGFR signaling could provide clinical benefit. Several receptor tyrosine kinase inhibitors active against PDGF/PDGFR signaling have been developed, including imatinib and vatalanib (described above). Imatinib is best characterized for its efficacy in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (89,90). While pre-clinical models showed that imatinib caused apoptosis in MPM cells lines and synergizes with gemcitabine (91), several phase I/II trials did not show efficacy imatinib monotherapy in patients with MPM. In a phase II study using imatinib at a dose of 200 mg BID, none of the 11 patients had a response (92) and, in another phase II study, none of the 17 MPM patients had a response to imatinib at a dose of 600 mg BID (93). Similarly, negative results were observed in a study in the Netherlands where 25 patients were treated with imatinib with doses ranging from 400 to 800 mg po BID without clinical response (94). The results of these three clinical trials show that single agent imatinib does not appear to offer clinical benefit to patients with MPM.
Several pre-clinical models indicated that imatinib reduces the interstitial pressure of tumor tissue and therefore could perhaps enhance delivery of other therapeutics (95). Further, in vitro studies demonstrate synergy between imatinib and gemcitabine or pemetrexed (96). With this rationale, a phase I study evaluated imatinib in combination with cisplatin and pemetrexed in 17 patients with MPM who had never received chemotherapy (97). Tsao et al. tested a regimen of cisplatin 75 mg/m2, pemetrexed 500 mg/m2, and imatinib mesylate 600 mg po BID; however, the regimen was only tolerable in good performance status patients. Seven out of the 17 patients received two cycles of therapy or less. By RECIST criteria, 1 patient had a partial response, and 3 had a minor response. Six patients were able to complete 6 cycles of chemotherapy and demonstrated a median progression free survival of 9.6 months and overall survival of 22.4 months. While this is higher compared to historical controls, this study was not designed to determine if the addition of imatinib to cisplatin and pemetrexed provided clinical benefit (97). In the setting of refractory MPM, a phase I study combined imatinib mesylate with gemcitabine where 1 patient out of 5 had a partial response (98). A phase II study is currently underway evaluating efficacy of imatinib in combination with gemcitabine in patients with MPM who had previously been treated (clinicaltrials.gov NCT02303899).
MET/HGF inhibitors
The mesenchymal-epidermal transition factor (MET) proto-oncogene encodes a receptor tyrosine kinase that, upon being bound by the ligand Hepatocyte Growth Factor (HGF), transduces signals from the extracellular matrix into the cytoplasm. This activates several signaling cascades including the RAS-ERK, PI3 kinase-AKT, or PLC gamma-PKC, which regulate physiological processes including proliferation, migration, and survival. Over-expression, amplification, and mutation in Met have been described in MPM cell lines and inhibition of MET reduces proliferation of MPM cells in vitro (99). However, there currently are no studies, to our knowledge, evaluating efficacy of MET inhibitors in MPM.
EGFR inhibitors
Prior studies reports that the percentage of MPM tumors that express epidermal growth factor receptor (EGFR) ranges from 32–97% (100-106). Preclinical studies demonstrate sensitivity of MPM cell lines to EGFR inhibitors (107). These findings have led to the hypothesis that targeting EGFR could be beneficial. There are two approaches to target EGFR which have been shown to be effective in patients with EGFR-mutated NSCLC, head and neck cancer, and RAS wild-type colon cancer. Small molecules, such as gefitinib and erlotinib, cross the plasma membrane of the targeted cell and bind to the intracellular domain of EGFR and inhibit EGFR signaling. Monoclonal antibodies, such as cetuximab, bind to the extracellular domain of EGFR and inhibit downstream signaling. Unfortunately, there is little evidence of efficacy from targeting EGFR in MPM. In a phase II study, none of the 63 MPM patients responded to single agent erlotinib in the front line setting despite high expression of EGFR (103). In a phase II trial evaluating single-agent gefitinib in the front line setting, only 2 out of 43 patients responded to therapy and thus the investigators of this study concluded that gefitinib is not active in MPM (104). A clinical trial evaluating cetuximab in combination with pemetrexed and either cisplatin or carboplatin in the front line setting is ongoing (clinicaltrials.gov NCT00996567).
PI3K/AKT/mTOR pathway inhibitors
Phosphatidylinositol-4,5-bisphosphate 3-kinases (PI3K)s are composed of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit that phosphorylates phosphatidyl inositol as a mechanism of intracellular signal transduction through mammalian target of rapamycin (mTOR)/AKT/ERK pathways regulating proliferation, differentiation, motility, and survival (108). Mutations in genes involved in PI3K signaling have been identified in MPM and are thought to contribute to the pathogenesis of MPM (109). Everolimus inhibits PI3K through binding mTOR and is FDA approved for metastatic breast cancer, renal cell carcinoma, subependymal giant cell astrocytoma, GI and lung neuroendocrine tumors, and for prevention of graft failure (110-113). Unfortunately, patients with MPM who had progressed after first line therapy did not respond to everolimus in a phase II study (114). Preclinical models have demonstrated promising efficacy in MPM patient derived xenograft models with a combination of everolimus and sorafenib (85) suggesting that the combination of receptor tyrosine kinase and mTOR inhibition could be effective. Other inhibitors of PI3K/mTOR are currently in clinical trials. A first in-human study is underway with the PI3K/mTOR inhibitor LY3023414, a small molecule that functions as a selective ATP-competitive inhibitor of PI3Kα and mTOR, DNA-PK in in vitro studies (clinicaltrials.gov NCT01655225). A phase I study evaluating safety and tolerability of apitolisib, a potent small molecule inhibit of PI3K and mTOR, reported a partial response in 2 out of 26 MPM patients (115). Recently, in vitro studies demonstrated that targeting MET and PI3Ks provides synergistic inhibition of MPM cell proliferation and migration, induction of apoptosis, and reduction in growth of an MPM patient derived xenograft mouse model (116), which provides a rationale for combinatorial therapy.
Targeting epigenetic regulators
A comprehensive genomic analysis identified mutations in BAP1 in 23% of MPM samples (117). Similarly, somatic mutations in BAP1 have been reported in approximately 20% of cases of MPM (118) and linkage analysis demonstrated that germ-line mutations in BAP1 are associated with familial MPM (16). The BAP1 gene encodes a ubiquitin hydrolase that functions as a catalytic unit of the polycomb repressive deubiquitinase complex crucial for regulating gene expression and facilitating DNA repair (119,120). The recurrent mutations in BAP1 prompted investigation into the use of histone deacetylate inhibitors in MPM. A phase I trial reported a partial response in 2/13 patients with MPM (121). The phase III clinical trial VANTAGE-014 sought to determine if vorinostat could improve overall survival as a 2nd or 3rd-line agent. On this trial, 661patients were enrolled across 90 international centers, and randomized in a double blind fashion to receive either vorinostat 300 mg or matching placebo twice daily on days 1, 2, 3, 8, 9, 10, 15, 16, and 17 of a 21-day cycle. The study found that median overall survival for patients who received vorinostat was 30.7 weeks (95% CI: 26.7–36.1) vs. 27.1 weeks (23.1–31.9) for placebo (hazard ratio 0.98, 95% CI: 0.83–1.17, P=0.86), demonstrating that vorinostat given as a second-line or third-line therapy did not improve overall survival (122). Similar clinical findings were noted for another histone deacetylase inhibitor, belinostat (123). While these clinical studies demonstrate lack of benefit after pharmacologic inhibition of histone deacetylase to target epigenetic regulation in MPM, a preclinical study identified synthetic lethality with pharmacologic inhibition of enhancer of zeste 2 polycomb repressive complex 2 subunit (Ezh2) in MPM cells lacking Bap1 (124). A phase II study evaluating efficacy of the EZH2 inhibitor tazemetostat in patients with both Bap1-deficient and wild-type relapsed or refractory MPM is currently ongoing (clinicaltrials.gov NCT02860286).
Focal adhesion kinase (FAK) inhibitors
NF-2 encodes the tumor suppressor Moesin-ezrin-radixin-like protein (Merlin), a protein that functions as a membrane-cytoskeleton scaffold. Merlin inhibits FAK, a ubiquitously expressed intracellular protein localized to areas where the cell membrane attaches to the extracellular matrix. FAK integrates signals from integrins, which are cell surface glycoproteins that interact with the extracellular matrix, into downstream effector pathways to regulate cell migration, adhesion, invasion, and self-renewal (125). Consequently, loss of NF-2/Merlin leads to tumorigenesis through dysregulation of migration which leads to an invasive phenotype. Germ-line loss of function mutations or deletions of NF2 cause neurofibromatosis, a disorder characterized by development of schwannomas, meningiomas, and ependymomas. Somatic mutations leading to loss of NF2 have been described in numerous cancers, including MPM. The recently published comprehensive genomic profiling of MPM identified mutations in NF-2 in 19% of cases (117).
Defactinib is a second generation small molecule inhibitor of FAK. Pre-clinical models demonstrated that maintenance therapy with defactinib delayed tumor regrowth in MPM patient derived xenograft models (126). In a phase I study, one patient with MPM was reported to have radiographic stable disease while on defactinib for 24 weeks at a dose of 400 mg (127). Interestingly, another phase I study with a different FAK inhibitor, GSK2256098, reported that MPM patients with documented loss of Merlin had increased median PFS at 23.4 weeks (n=14) compared to MPM patients with preserved Merlin expression who had median PFS at 11.4 weeks (n=9) (128). This study was not designed to show a statistically significant difference in PFS but suggested that further studies could consider patient stratification based on NF2 mutations status or levels of Merlin expression. Overall, these studies provided a rationale for a phase IIb study to evaluate defactinib as maintenance therapy in MPM patients after first line therapy with pemetrexed and a platinum agent. Unfortunately, this phase II study has been terminated after enrollment of 344 patients due to lack of observed efficacy (COMMAND, clinicaltrials.gov NCT01870609).
Hsp90 inhibition
Heat shock proteins (HSPs) constitute a large family of proteins involved in protein folding and maturation. The major groups of HSPs are classified based on molecular weight of which HSP90 has been implicated in multiple malignancy types (129). Targeting HSP90 is an attractive therapeutic strategy as HSP90 functions to stabilize and fold multiple client proteins involved in cancer cell signaling such as EGFR, IGF-1R, CDK4, AKT, ErbB2, c-Met, BCR-ABL, RET, androgen receptors, Fms-like tyrosine kinase 3 (FLT3), BRAF, NF-κB, Raf-1, HER2/Neu, NPM-ALK, p53, neuronal nitric oxide synthase (nNOS), and HIF-1α (129). Ganetespib binds to and inhibits HSP90, resulting in the proteasomal degradation of oncogenic client proteins (130). A phase I/II clinical trial (MESO-02, clinicaltrials.gov NCT01590160) evaluating ganetespib with platinum in the front-line setting has completed accrual and results are pending.
Proteasome inhibition
The potent proteasome 20S inhibitor bortezomib is approved for use in multiple myeloma (131). The mechanism through which bortezomib inhibition of the proteasome leads to cancer cell death is not fully understood but is thought that disruption of the single proteasome target affects multiple signaling pathways. Bortezomib induces cell cycle arrest and is synergistic with cisplatin in MPM cell line in vitro (132). In the second line setting, only 1 out of 23 patients treated with bortezomib and cisplatin demonstrated a partial response in a single arm phase II study (133). In the front-line setting, the response rate to bortezomib combined with cisplatin in MPM was reported at 28.4% (95% CI: 18.9–39.5%) with a median overall survival of 13.5 months (85% CI: 10.5–15 months). Unfortunately, the progression free survival at 18 weeks was 53% (80% CI: 42–64%) which was below the threshold to predict success of cisplatin and bortezomib in a phase III study and there are no further plans to study bortezomib with cisplatin (134).
Immuno-targeting of mesothelin
Chang et al. first identified mesothelin as the antigen of the K1 monoclonal antibody generated from mice immunized with an ovarian cancer cell line (135). The mesothelin gene encodes a 69 kD protein which is cleaved into a 40 kD and 32 kD fragment. The 40 kD fragment is anchored to the surface of mesothelial cells lining the pleura by a GPI-linked membrane-bound protein. MPM expresses mesothelin at high levels since this malignancy arise from normal mesothelial cells. The physiologic function of mesothelin is unknown and mice lacking mesothelin do not have a phenotype (136). The high expression of mesothelin on MPM compared to normal tissue implies that mesothelin could be an effective target for immune guided therapies. Against this background, multiple anti-mesothelin therapeutic strategies have been developed including the monoclonal antibody amatuximab, an antibody-drug conjugates with the fully human anti-mesothelin antibody intetumumab (NFT), and recombinant immunotoxins.
Amatuximab
The mouse-human chimeric IgG1k monoclonal antibody amatuximab has a binding affinity of 1.5 nM for human mesothelin (137). Two phase I clinical trials identified a maximum tolerated dose of 200 mg/m2; however, no responses were seen in patients with MPM who received this agent (138,139). In a phase II study, amatuximab combined with pemetrexed and cisplatin in patients with unresectable epithelioid MPM did not show overlapping toxicities (140). The addition of amatuximab to pemetrexed and cisplatin did not prolong PFS longer than historical controls; however, median OS was 14.8 months compared to the historical control of 13.3 months for pemetrexed and cisplatin along. A phase II double blind placebo controlled study in six centers is ongoing (clinicaltrials.gov NCT02357147).
Antibody-drug conjugates
There are currently three different antibody-drug conjugates targeting mesothelin expressing cells in clinical trials (141). The concept of a “magic bullet” delivering a potent chemotherapeutic to cancer cells and not normal healthy cells was first proposed over a century ago (142). Today, standard of care oncology practice utilizes antibody-drug conjugates with the approval of trastuzumab-emtansine (TDM1) (143) and brentuxumab vedotin (144-146). Antibody drug conjugates seek to utilize the specificity of antibody affinity to deliver highly potent cytotoxic agents specifically to cancer cells. While conceptually simple, the effectiveness of an antibody-drug conjugate depends on the effectiveness of the antibody, drug, and the linker fastening the drug to the antibody. After systemic administration, the antibody-drug conjugate binds the targeted epitope on the surface of the cancer cell which then internalizes the antibody-drug conjugate by receptor mediated endocytosis (147). Once internalized, the cytotoxic drug is released from the antibody through either hydrolysis, enzymatic cleavage, of degradation of the antibody depending on the linker used.
Anetumab ravtansine is currently undergoing clinical testing in MPM. Phage library display panning led to the identification of Fab MF-T which binds mesothelin with 10 nM affinity (148). The conjugation of MF-T to the maytansinoid tubulin inhibitor DM4 through a hindered disulfide linker generated the antibody-drug conjugate anetumab ravtansine (BAY94-9343) (148). The uptake of the DM4 toxin causes inhibition of mitosis through targeting microtubule polymerization (149). Further, neighboring cells are also affected through a phenomenon known as “bystander cytotoxicity” where active drug metabolites diffuse into neighboring cells (150). A phase I study of intravenously infused anetumab ravtansine every 3 weeks in 147 cancer patients identified a maximum tolerated dose of 6.5 mg/kg. The investigators identified keratopathy, asymptomatic increase in serum aminotransferase levels, and gastrointestinal upset as adverse events. In a subset of patients with MPM treated with anetumab ravtansine at the maximum tolerated dose of 6.5 mg/kg i.v. every 3 weeks (n=16), 31% of patients had a partial response and 44% of patients has stable disease for an overall disease control rate of 75% (151) (clinicaltrials.gov NCT01439152) . Encouraged by these results, these investigators are currently performing a phase II trial in 2nd-line metastatic pleural mesothelioma (clinicaltrials.gov NCT02610140).
Using antibody-drug conjugate technology, the humanized mouse anti-mesothelin antibody 7D9 was linked to monomethyl auristatin E via a lysosomal protease-cleavable valine-citrulline dipeptide linker, and has demonstrated promising activity in preclinical models (152). A phase Ia/II study of a mesothelin-directed antibody drug conjugate with an undisclosed cytotoxic drug (BMS-986148) was initiated in patients with advanced solid tumors, including mesothelioma, and this study is currently recruiting patients for enrollment (clinicaltrials.gov NCT02341625).
Recombinant immunotoxins
Two novel agents, SS1P and RG7787 (LMB-100), link anti-mesothelin moieties to portions of Pseudomonas endotoxin A (141). These agents have potent efficacy in vitro (153) and the anti-tumor effect is enhanced in mouse models with prior administration of paclitaxel which is thought to decrease interstitial tumor pressure and increases tumor uptake of recombinant immunotoxin (154-156). Phase I studies have shown that both bolus and infusional doses of SS1P were complicated by generation of neutralizing antibodies to the pseudomonas endotoxin (157,158). Hassan et al. markedly delayed the development of these neutralizing antibodies by administering pentostatin and cyclophosphamide before and during administration of SS1P to deplete T and B lymphocytes. Of the 10 patients with chemotherapy-refractory MPM, 3 had major tumor regression and antibody formation was markedly delayed, allowing for more cycles to be given (159). Hollevoet et al. de-immunized the effector moiety of the pseudomonas endotoxin A and fused it with a mesothelin targeting moiety to generated RG7787 (156). This recombinant immunotoxin has activity in MPM patient derived xenograft models (160). RG7787 was renamed LMB-100 and there is currently a phase I study accruing patients to evaluate the maximum tolerated dose of RG7787/LMB-100 in patients with MPM (clinicaltrials.gov NCT02798536).
Arginine depletion
Preclinical models demonstrated that arginine deprivation is synthetically lethal in MPM cells that do not express argininosuccinate synthetase 1 (ASS1) (161). Approximately 60% of MPM tumors have loss of ASS1. Ubiquitous expression of ASS1 in normal cells offers a wide therapeutic window for arginine depletion therapy as the toxicity to normal cells is low. A phase II multicenter study compared administration of pegylated arginine deiminase (Adi-PEG 20, 36.8 mg/m2 i.m. weekly) in addition to best supportive care to best supportive care alone in patients with ASS1 negative MPM (162). The study enrolled 201 patients, of which 97 were found to have ASS1 deficient disease as defined by >50% low expressing cells as visualized by an anti-ASS1 antibody. As an aside, the investigators were unable to determine the ASS1 status of 21 patients and the remaining 83 ASS1 positive patients were analyzed for overall survival. Szlosarek et al. reported a PFS hazard ratio of 0.56 (95% CI: 0.33–0.96) with a median PFS of 3.2 months in the Adi-PEG 20 group compared to 2.0 months in the best supportive care group alone (P=0.03) (162). The authors observed nonfebrile neutropenia, gastrointestinal toxicity, and fatigue as the most common adverse events. A phase I dose escalation study which combined Adi-PEG 20 with cisplatin and pemetrexed provided a signal of efficacy with a 78% response rate (7 out of 9 patients) (163). These findings are consistent with demonstrated efficacy of arginine depletion therapy in other malignancies (164-167). A phase 2/3 study is currently recruiting patients with MPM with low ASS1 expression to evaluate the efficacy of Adi PEG 20 in combination with pemetrexed and cisplatin (clinicaltrials.gov NCT02709512).
Immunotherapy
The advances in immunotherapeutic approaches for MPM are reviewed in more detail elsewhere in this issue of Translational Lung Cancer Research. However, highlights of recent immunotherapy trials will be presented here. The immune compartment has proven to be a key component of the tumor microenvironments role in tumor initiation, progression, and response to therapy (168). Targeting molecular regulators of immune function, namely cytotoxic lymphocyte antigen 4 (CTLA4) and Programmed Death-1/Programmed Death-Ligand 1 (PD/PD-L1) signaling axis have emerged as effective therapeutic strategies in multiple cancers (169). Recent efforts have focused on determining if MPM is responsive to immunotherapy. MPM has a high variability of lymphocytic infiltration, but prolonged survival is associated with a higher presence of lymphoid cells (170). Additionally, PD-L1 expression is variable, is associated with populations of infiltrating T cells, and may be more associated with sarcomatoid histology and a worse prognosis (171-174). Multiple clinical trials have sought to determine or are currently determining if MPM responds to immune mediated therapies (Table 2).
CTLA4 inhibition
Tremelimumab is a selective human IgG2 monoclonal function blocking antibody against CTLA4 that blocks interaction with B7 and promotes anti-tumor effects of tumor infiltrating leukocytes (169). In the second-line setting, 2 out of 29 patients with MPM (7%) demonstrated a durable partial response to tremelimumab 15 mg/kg every 90 days (MESOT-TREM-2008) (175). Retrospective exposure-response analysis of data from melanoma suggested the dosing schedule of tremelimumab 15 mg/kg every 90 days resulted in underexposure to tremelimumab. A single-arm phase 2 study was then performed to evaluate the efficacy of tremelimumab 10 mg/kg every 4 weeks for 6 doses, followed by dosing every 12 weeks until disease progression. Four out of 29 patients were found to have a response and 15 out of 29 patients were found to have disease control with median duration of 10.9 months (95% CI: 8.2–13.6). Unfortunately, results of the double blind, placebo controlled DETERMINE (clinicaltrials.gov NCT01843374) study showed tremelimumab monotherapy is not superior to placebo for the primary endpoint of overall survival (tremelimumab vs. placebo median OS 7.7 vs. 7.3 mo; HR =0.92; 95% CI: 0.76–1.12, P=0.408) (176). Clinical studies that determine the efficacy of combining CTLA4 blockade with PD-1/PD-L1 blockade are ongoing (Table 2, clinicaltrials.gov NCT02899299 and NCT02588131).
Programmed death-1/programmed death ligand-1 inhibition
T lymphocytes express PD-1 which, upon binding to PD-L1 or PD-L2 on the surface of a potential target cell, inhibits cytotoxic killing of that target cell (169). MPM cells may inhibit the anti-tumor immune response and evade T lymphocyte mediated cell killing by expression of PD-L1 (169). Monoclonal antibodies against PD-1 (pembrolizumab and nivolumab) or PD-L1 (avelumab, atezolizumab, durvalumab) block the interaction of tumor cell expressed PD-L1 with PD-1 on the surface of T lymphocytes and thereby aim to inhibit the anti-tumor immune response. The Keynote-028 trial reported that 7 out of 25 PD-L1 positive MPM patients had a partial response to monotherapy with pembrolizumab (10 mg/kg every 2 weeks). The overall response rate of 28% and disease control rate of 76% are better than expected in the second line setting (177). In a single center phase II study, patients with MPM were treated in the second line setting with nivolumab 3 mg/kg every two weeks until progression or toxicity. Five out of 38 patients had a partial response and the disease control rate was 50% at 12 weeks (178). Lastly, a phase I study evaluating safety and treatment related toxicity of avelumab, a fully human anti-PD-L1 IgG antibody, reported an overall response rate of 14.3% in PD-L1 positive patients with unresectable MPM, and a response rate of 8% in patients with MPM without PD-L1 expression (179). These results indicate that targeting the PD-1/PD-L1 axis in MPM appears promising. Phase III trials evaluating PD-1/PD-L1 blockade in the setting of first-line therapy for MPM or relapsed disease and clinical studies combining CTLA4 inhibitors (above) with PD-1/PD-L1 blockade in patient with MPM are currently ongoing (Table 2). Further, efforts to identify predictors of response to immunotherapy, such as tumor molecular features or characterization of tumor immune infiltrates, could inform patient selection for immunotherapy.
Conclusions
To date, the combination of pemetrexed, either cisplatin or carboplatin, and the optional addition of bevacizumab, is standard therapy for MPM in the frontline setting. Currently, there is no approved therapy for refractory disease, but gemcitabine and vinorelbine have activity in some patients, and immunotherapy is increasingly used off-label. Even with systemic therapy, median overall survival is approximately one year and the chance of long-term survival is low. Many approaches are being pursued to evaluate novel therapeutic strategies to improve response rates, identify second-line therapies, determine if maintenance therapy is beneficial, and develop optimal regimens for the elderly and frail. The lack of “single driver mutations” identified in MPM presents a challenge to the development of molecular targeted therapies for MPM and highlights a need for a better understanding of MPM biology towards developing personalized approaches to therapy. Furthermore, the heterogeneity of MPM, the relatively low incidence of the disease, and the challenge to assess radiographic and clinical response to therapy pose barriers to developing more effective systemic therapies. Despite these challenges, mesothelin targeting, arginine depletion, and immunotherapy appear to be among the most promising of the emerging therapeutic strategies.
Acknowledgements
None.
Footnote
Conflicts of Interest: MR Mancuso has no conflicts of interest to declare. JW Neal has consulting or advisory roles with Clovis Oncology, Boehringer Ingelheim, ARMO Biosciences, Nektar Therapeutics, ARIAD pharmaceuticals/Takeda, Eli Lilly and Company, and Physician Resource Management. JW Neal receives research funding from Genentech/Roche, Merck, ArQule, Novartis, Boehringer Ingelheim, Nektar, and ARIAD. JW Neal does not have conflicts of interest to declare relating to employment, leadership, stocks and ownership interests, honoraria, patents, royalties, or other intellectual property, expert testimony, or receiving of travel expenses.
References
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88. [Crossref] [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Women with malignant pleural mesothelioma have a threefold better survival rate than men. Ann Thorac Surg 2014;98:1020-4. [Crossref] [PubMed]
- Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980;46:2736-40. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [PubMed]
- Nishikawa K, Takahashi K, Karjalainen A, et al. Recent mortality from pleural mesothelioma, historical patterns of asbestos use, and adoption of bans: a global assessment. Environ Health Perspect 2008;116:1675-80. [Crossref] [PubMed]
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8. [Crossref] [PubMed]
- Carbone M, Baris YI, Bertino P, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A 2011;108:13618-23. [Crossref] [PubMed]
- Cavazza A, Travis LB, Travis WD, et al. Post-irradiation malignant mesothelioma. Cancer 1996;77:1379-85. [Crossref] [PubMed]
- De Bruin ML, Burgers JA, Baas P, et al. Malignant mesothelioma after radiation treatment for Hodgkin lymphoma. Blood 2009;113:3679-81. [Crossref] [PubMed]
- Deutsch M, Land SR, Begovic M, et al. An association between postoperative radiotherapy for primary breast cancer in 11 National Surgical Adjuvant Breast and Bowel Project (NSABP) studies and the subsequent appearance of pleural mesothelioma. Am J Clin Oncol 2007;30:294-6. [Crossref] [PubMed]
- Goodman JE, Nascarella MA, Valberg PA. Ionizing radiation: a risk factor for mesothelioma. Cancer Causes Control 2009;20:1237-54. [Crossref] [PubMed]
- Li X, Brownlee NA, Sporn TA, et al. Malignant (Diffuse) Mesothelioma in Patients With Hematologic Malignancies: A Clinicopathologic Study of 45 Cases. Arch Pathol Lab Med 2015;139:1129-36. [Crossref] [PubMed]
- Teta MJ, Lau E, Sceurman BK, et al. Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer 2007;109:1432-8. [Crossref] [PubMed]
- Witherby SM, Butnor KJ, Grunberg SM. Malignant mesothelioma following thoracic radiotherapy for lung cancer. Lung Cancer 2007;57:410-3. [Crossref] [PubMed]
- Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5. [Crossref] [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Patel SC, Dowell JE. Modern management of malignant pleural mesothelioma. Lung Cancer (Auckl) 2016;7:63-72. [PubMed]
- Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993;72:394-404. [Crossref] [PubMed]
- Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J 1998;11:213-21. [Crossref] [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [Crossref] [PubMed]
- Adams RF, Gray W, Davies RJ, et al. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798-802. [Crossref] [PubMed]
- Beauchamp HD, Kundra NK, Aranson R, et al. The role of closed pleural needle biopsy in the diagnosis of malignant mesothelioma of the pleura. Chest 1992;102:1110-2. [Crossref] [PubMed]
- Boutin C, Schlesser M, Frenay C, et al. Malignant pleural mesothelioma. Eur Respir J 1998;12:972-81. [Crossref] [PubMed]
- Chakrabarti B, Ryland I, Sheard J, et al. The role of Abrams percutaneous pleural biopsy in the investigation of exudative pleural effusions. Chest 2006;129:1549-55. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Hjerpe A, Ascoli V, Bedrossian C, et al. Guidelines for cytopathologic diagnosis of epithelioid and mixed type malignant mesothelioma. Complementary statement from the International Mesothelioma Interest Group, also endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology. Cytojournal 2015;12:26. [Crossref] [PubMed]
- Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2005;63:1045-52. [Crossref] [PubMed]
- Baldini EH, Richards WG, Gill RR, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015;149:1374-81. [Crossref] [PubMed]
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80; discussion 580-2. [Crossref] [PubMed]
- Linton A, Pavlakis N, O'Connell R, et al. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. Br J Cancer 2014;111:1860-9. [Crossref] [PubMed]
- Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015;196:23-32. [Crossref] [PubMed]
- Musk AW, Olsen N, Alfonso H, et al. Predicting survival in malignant mesothelioma. Eur Respir J 2011;38:1420-4. [Crossref] [PubMed]
- Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081-90. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Cantwell BM, Franks CR, Harris AL. A phase II study of the platinum analogues JM8 and JM9 in malignant pleural mesothelioma. Cancer Chemother Pharmacol 1986;18:286-8. [Crossref] [PubMed]
- Chahinian AP, Antman K, Goutsou M, et al. Randomized phase II trial of cisplatin with mitomycin or doxorubicin for malignant mesothelioma by the Cancer and Leukemia Group B. J Clin Oncol 1993;11:1559-65. [Crossref] [PubMed]
- Ellis P, Davies AM, Evans WK, et al. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: a systematic review and practice guideline. J Thorac Oncol 2006;1:591-601. [Crossref] [PubMed]
- Samson MK, Wasser LP, Borden EC, et al. Randomized comparison of cyclophosphamide, imidazole carboxamide, and adriamycin versus cyclophosphamide and adriamycin in patients with advanced stage malignant mesothelioma: a Sarcoma Intergroup Study. J Clin Oncol 1987;5:86-91. [Crossref] [PubMed]
- Sørense PG, Bach F, Bork E, et al. Randomized trial of doxorubicin versus cyclophosphamide in diffuse malignant pleural mesothelioma. Cancer Treat Rep 1985;69:1431-2. [PubMed]
- Berghmans T, Paesmans M, Lalami Y, et al. Activity of chemotherapy and immunotherapy on malignant mesothelioma: a systematic review of the literature with meta-analysis. Lung Cancer 2002;38:111-21. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Hughes A, Calvert P, Azzabi A, et al. Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 2002;20:3533-44. [Crossref] [PubMed]
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3. [Crossref] [PubMed]
- Ceresoli GL, Castagneto B, Zucali PA, et al. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer 2008;99:51-6. [Crossref] [PubMed]
- Katirtzoglou N, Gkiozos I, Makrilia N, et al. Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: a phase II study. Clin Lung Cancer 2010;11:30-5. [Crossref] [PubMed]
- Santoro A, O'Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008;3:756-63. [Crossref] [PubMed]
- Lee CW, Murray N, Anderson H, et al. Outcomes with first-line platinum-based combination chemotherapy for malignant pleural mesothelioma: a review of practice in British Columbia. Lung Cancer 2009;64:308-13. [Crossref] [PubMed]
- Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol 1999;17:25-30. [Crossref] [PubMed]
- Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491-6. [Crossref] [PubMed]
- Kalmadi SR, Rankin C, Kraut MJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: a phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 2008;60:259-63. [Crossref] [PubMed]
- Ak G, Metintas S, Akarsu M, et al. The effectiveness and safety of platinum-based pemetrexed and platinum-based gemcitabine treatment in patients with malignant pleural mesothelioma. BMC Cancer 2015;15:510. [Crossref] [PubMed]
- Favaretto AG, Aversa SM, Paccagnella A, et al. Gemcitabine combined with carboplatin in patients with malignant pleural mesothelioma: a multicentric phase II study. Cancer 2003;97:2791-7. [Crossref] [PubMed]
- Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol 2005;28:223-6. [Crossref] [PubMed]
- van Haarst JM, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer 2002;86:342-5. [Crossref] [PubMed]
- Steele JP, Shamash J, Evans MT, et al. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 2000;18:3912-7. [Crossref] [PubMed]
- Fennell DA. Phase II trial of vinorelbine and oxaliplatin as first-line therapy in malignant pleural mesothelioma. Lung Cancer 2005;47:277-81. [Crossref] [PubMed]
- Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94-7. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Ray M, Kindler HL. Malignant Pleural Mesothelioma: An Update on Biomarkers and Treatment. Chest 2009;136:888-96. [Crossref] [PubMed]
- Vogelzang NJ. Chemotherapy for malignant pleural mesothelioma. Lancet 2008;371:1640-2. [Crossref] [PubMed]
- Manegold C, Symanowski J, Gatzemeier U, et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol 2005;16:923-7. [Crossref] [PubMed]
- Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008;26:1698-704. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, Gianoncelli L, et al. Second-line treatment for malignant pleural mesothelioma. Cancer Treat Rev 2010;36:24-32. [Crossref] [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Jing XQ, Zhou L, Sun XD, et al. Pemetrexed Maintenance Therapy Following Bevacizumab-Containing First-Line Chemotherapy in Advanced Malignant Pleural Mesothelioma: A Case Report and Literatures Review. Medicine (Baltimore) 2016;95:e3351. [Crossref] [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [Crossref] [PubMed]
- D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 1994;91:4082-5. [Crossref] [PubMed]
- Remon J, Reguart N, Corral J, et al. Malignant pleural mesothelioma: new hope in the horizon with novel therapeutic strategies. Cancer Treat Rev 2015;41:27-34. [Crossref] [PubMed]
- Buikhuisen WA, Scharpfenecker M, Griffioen AW, et al. A Randomized Phase II Study Adding Axitinib to Pemetrexed-Cisplatin in Patients with Malignant Pleural Mesothelioma: A Single-Center Trial Combining Clinical and Translational Outcomes. J Thorac Oncol 2016;11:758-68. [Crossref] [PubMed]
- Grosso F, Steele N, Novello S, et al. Nintedanib plus pemetrexed/cisplatin in patients with MPM: Phase II findings from the placebo-controlled LUME-Meso trial. Vienna: 17th IASLC World Conference on Lung Cancer 2016 Contract No.: ID #4191
- Heckman CA, Holopainen T, Wirzenius M, et al. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res 2008;68:4754-62. [Crossref] [PubMed]
- Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 2005;65:4389-400. [Crossref] [PubMed]
- Campbell NP, Kunnavakkam R, Leighl N, et al. Cediranib in patients with malignant mesothelioma: a phase II trial of the University of Chicago Phase II Consortium. Lung Cancer 2012;78:76-80. [Crossref] [PubMed]
- Garland LL, Chansky K, Wozniak AJ, et al. Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thorac Oncol 2011;6:1938-45. [Crossref] [PubMed]
- Tsao AS, Moon J, Moon II, et al. A phase I study of cediranib (NSC #732208) in combination with cisplatin and pemetrexed in chemonaive patients with malignant pleural mesothelioma (SWOG S0905). J Clin Oncol 2013;31 suppl:abstr 7527.
- Laurie SA, Gupta A, Chu Q, et al. Brief report: a phase II study of sunitinib in malignant pleural mesothelioma. the NCIC Clinical Trials Group. J Thorac Oncol 2011;6:1950-4. [Crossref] [PubMed]
- Jahan T, Gu L, Kratzke R, et al. Vatalanib in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (CALGB 30107). Lung Cancer 2012;76:393-6. [Crossref] [PubMed]
- Dubey S, Janne PA, Krug L, et al. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol 2010;5:1655-61. [Crossref] [PubMed]
- Papa S, Popat S, Shah R, et al. Phase 2 study of sorafenib in malignant mesothelioma previously treated with platinum-containing chemotherapy. J Thorac Oncol 2013;8:783-7. [Crossref] [PubMed]
- Pignochino Y, Dell'Aglio C, Inghilleri S, et al. The combination of sorafenib and everolimus shows antitumor activity in preclinical models of malignant pleural mesothelioma. BMC Cancer 2015;15:374. [Crossref] [PubMed]
- Kothmaier H, Quehenberger F, Halbwedl I, et al. EGFR and PDGFR differentially promote growth in malignant epithelioid mesothelioma of short and long term survivors. Thorax 2008;63:345-51. [Crossref] [PubMed]
- Langerak AW, De Laat PA, Van Der Linden-Van Beurden CA, et al. Expression of platelet-derived growth factor (PDGF) and PDGF receptors in human malignant mesothelioma in vitro and in vivo. J Pathol 1996;178:151-60. [Crossref] [PubMed]
- Li Q, Wang W, Yamada T, et al. Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am J Pathol 2011;179:1483-93. [Crossref] [PubMed]
- Dagher R, Cohen M, Williams G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res 2002;8:3034-8. [PubMed]
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031-7. [Crossref] [PubMed]
- Vogelzang NJ, Porta C, Mutti L. New agents in the management of advanced mesothelioma. Semin Oncol 2005;32:336-50. [Crossref] [PubMed]
- Porta C, Mutti L, Tassi G. Negative results of an Italian Group for Mesothelioma (G.I.Me.) pilot study of single-agent imatinib mesylate in malignant pleural mesothelioma. Cancer Chemother Pharmacol 2007;59:149-50. [Crossref] [PubMed]
- Villano JL, Husain AN, Stadler WM, et al. A Phase II trial of imatinib mesylate in patients (pts) with malignant mesothelioma (MM). J Clin Oncol 2004.22. Abstract 7200.
- Mathy A, Baas P, Dalesio O, et al. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer 2005;50:83-6. [Crossref] [PubMed]
- Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 2002;62:5476-84. [PubMed]
- Bertino P, Porta C, Barbone D, et al. Preliminary data suggestive of a novel translational approach to mesothelioma treatment: imatinib mesylate with gemcitabine or pemetrexed. Thorax 2007;62:690-5. [Crossref] [PubMed]
- Tsao AS, Harun N, Lee JJ, et al. Phase I trial of cisplatin, pemetrexed, and imatinib mesylate in chemonaive patients with unresectable malignant pleural mesothelioma. Clin Lung Cancer 2014;15:197-201. [Crossref] [PubMed]
- Ali Y, Lin Y, Gharibo MM, et al. Phase I and pharmacokinetic study of imatinib mesylate (Gleevec) and gemcitabine in patients with refractory solid tumors. Clin Cancer Res 2007;13:5876-82. [Crossref] [PubMed]
- Jagadeeswaran R, Ma PC, Seiwert TY, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res 2006;66:352-61. [Crossref] [PubMed]
- Dazzi H, Hasleton PS, Thatcher N, et al. Malignant pleural mesothelioma and epidermal growth factor receptor (EGF-R). Relationship of EGF-R with histology and survival using fixed paraffin embedded tissue and the F4, monoclonal antibody. Br J Cancer 1990;61:924-6. [Crossref] [PubMed]
- Destro A, Ceresoli GL, Falleni M, et al. EGFR overexpression in malignant pleural mesothelioma. An immunohistochemical and molecular study with clinico-pathological correlations. Lung Cancer 2006;51:207-15. [Crossref] [PubMed]
- Edwards JG, Swinson DE, Jones JL, et al. EGFR expression: associations with outcome and clinicopathological variables in malignant pleural mesothelioma. Lung Cancer 2006;54:399-407. [Crossref] [PubMed]
- Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 2007;25:2406-13. [Crossref] [PubMed]
- Govindan R, Kratzke RA, Herndon JE 2nd, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res 2005;11:2300-4. [Crossref] [PubMed]
- O'Byrne KJ, Edwards JG, Waller DA. Clinico-pathological and biological prognostic factors in pleural malignant mesothelioma. Lung Cancer 2004;45 Suppl 1:S45-8. [Crossref] [PubMed]
- Okuda K, Sasaki H, Kawano O, et al. Epidermal growth factor receptor gene mutation, amplification and protein expression in malignant pleural mesothelioma. J Cancer Res Clin Oncol 2008;134:1105-11. [Crossref] [PubMed]
- Jänne PA, Taffaro ML, Salgia R, et al. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res 2002;62:5242-7. [PubMed]
- Rodgers SJ, Ferguson DT, Mitchell CA, et al. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci Rep 2017.37. [PubMed]
- Lo Iacono M, Monica V, Righi L, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol 2015;10:492-9. [Crossref] [PubMed]
- Afriansyah A, Hamid AR, Mochtar CA, et al. Targeted Therapy for Metastatic Renal Cell Carcinoma. Acta Med Indones 2016;48:335-47. [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Jancic J, Duric V, Ivancevic N, et al. Current Use of mTOR Inhibitors for the Treatment of Subependymal Giant Cell Astrocytomas and Epilepsy in Patients with TSC. Curr Med Chem 2016;23:4260-9. [Crossref] [PubMed]
- Ohmoto A, Rokutan H, Yachida S. Pancreatic Neuroendocrine Neoplasms: Basic Biology, Current Treatment Strategies and Prospects for the Future. Int J Mol Sci 2017.18. [PubMed]
- Ou SH, Moon J, Garland LL, et al. SWOG S0722: phase II study of mTOR inhibitor everolimus (RAD001) in advanced malignant pleural mesothelioma (MPM). J Thorac Oncol 2015;10:387-91. [Crossref] [PubMed]
- Dolly SO, Wagner AJ, Bendell JC, et al. Phase I Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res 2016;22:2874-84. [Crossref] [PubMed]
- Kanteti R, Riehm JJ, Dhanasingh I, et al. PI3 Kinase Pathway and MET Inhibition is Efficacious in Malignant Pleural Mesothelioma. Sci Rep 2016;6:32992. [Crossref] [PubMed]
- Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407-16. [Crossref] [PubMed]
- Zauderer MG, Bott M, McMillan R, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 2013;8:1430-3. [Crossref] [PubMed]
- Jensen DE, Proctor M, Marquis ST, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16:1097-112. [Crossref] [PubMed]
- Sowa ME, Bennett EJ, Gygi SP, et al. Defining the human deubiquitinating enzyme interaction landscape. Cell 2009;138:389-403. [Crossref] [PubMed]
- Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 2005;23:3923-31. [Crossref] [PubMed]
- Krug LM, Kindler HL, Calvert H, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2015;16:447-56. [Crossref] [PubMed]
- Ramalingam SS, Belani CP, Ruel C, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol 2009;4:97-101. [Crossref] [PubMed]
- LaFave LM, Beguelin W, Koche R, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344-9. [Crossref] [PubMed]
- Petrilli AM, Fernandez-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016;35:537-48. [Crossref] [PubMed]
- Pachter JA, Kolev VN, Schunselaar L, et al. Abstract 4236: FAK inhibitor VS-6063 (defactinib) targets mesothelioma cancer stem cells, which are enriched by standard of care chemotherapy. Cancer Res 2015.75. Abstract 4236.
- Shimizu T, Fukuoka K, Takeda M, et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2016;77:997-1003. [Crossref] [PubMed]
- Soria JC, Gan HK, Blagden SP, et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol 2016;27:2268-74. [Crossref] [PubMed]
- Wu J, Liu T, Rios Z, et al. Heat Shock Proteins and Cancer. Trends Pharmacol Sci 2017;38:226-56. [Crossref] [PubMed]
- Wang Y, Trepel JB, Neckers LM, et al. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs 2010;11:1466-76. [PubMed]
- Curran MP, McKeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs 2009;69:859-88. [Crossref] [PubMed]
- Wang Y, Rishi AK, Puliyappadamba VT, et al. Targeted proteasome inhibition by Velcade induces apoptosis in human mesothelioma and breast cancer cell lines. Cancer Chemother Pharmacol 2010;66:455-66. [Crossref] [PubMed]
- Fennell DA, McDowell C, Busacca S, et al. Phase II clinical trial of first or second-line treatment with bortezomib in patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:1466-70. [Crossref] [PubMed]
- O'Brien ME, Gaafar RM, Popat S, et al. Phase II study of first-line bortezomib and cisplatin in malignant pleural mesothelioma and prospective validation of progression free survival rate as a primary end-point for mesothelioma clinical trials (European Organisation for Research and Treatment of Cancer 08052). Eur J Cancer 2013;49:2815-22. [Crossref] [PubMed]
- Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 1996;93:136-40. [Crossref] [PubMed]
- Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 2000;20:2902-6. [Crossref] [PubMed]
- Chowdhury PS, Viner JL, Beers R, et al. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci U S A 1998;95:669-74. [Crossref] [PubMed]
- Fujisaka Y, Kurata T, Tanaka K, et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs 2015;33:380-8. [Crossref] [PubMed]
- Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]
- Zhao XY, Subramanyam B, Sarapa N, et al. Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors. Clin Cancer Drugs 2016;3:76-86. [Crossref] [PubMed]
- Perez HL, Cardarelli PM, Deshpande S, et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today 2014;19:869-81. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Five-Year Survival Data Demonstrating Durable Responses From a Pivotal Phase 2 Study of Brentuximab Vedotin in Patients With Relapsed or Refractory Hodgkin Lymphoma. Clin Adv Hematol Oncol 2016;14:6. [PubMed]
- Updated Efficacy and Safety Data From the AETHERA Trial of Consolidation With Brentuximab Vedotin After Autologous Stem Cell Transplant (ASCT) in Hodgkin Lymphoma Patients at High Risk of Relapse. Clin Adv Hematol Oncol 2016;14:17-8.
- Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;385:1853-62. [Crossref] [PubMed]
- Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 2013;5:13-21. [Crossref] [PubMed]
- Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014;13:1537-48. [Crossref] [PubMed]
- Kupchan SM, Komoda Y, Court WA, et al. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc 1972;94:1354-6. [Crossref] [PubMed]
- Kovtun YV, Audette CA, Ye Y, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 2006;66:3214-21. [Crossref] [PubMed]
- Blumenschein GR, Hassan R, Moore KN, et al. Phase I study of anti-mesothelin antibody drug conjugate anetumab ravtansine (AR). J Clin Oncol 2016;34:abstr 2509.
- Scales SJ, Gupta N, Pacheco G, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther 2014;13:2630-40. [Crossref] [PubMed]
- Zhang Y, Xiang L, Hassan R, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res 2006;12:4695-701. [Crossref] [PubMed]
- Griffon-Etienne G, Boucher Y, Brekken C, et al. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res 1999;59:3776-82. [PubMed]
- Holden SA, Lan Y, Pardo AM, et al. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin Cancer Res 2001;7:2862-9. [PubMed]
- Hollevoet K, Mason-Osann E, Liu XF, et al. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther 2014;13:2040-9. [Crossref] [PubMed]
- Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144-9. [Crossref] [PubMed]
- Kreitman RJ, Hassan R, Fitzgerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009;15:5274-9. [Crossref] [PubMed]
- Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013;5:208ra147. [Crossref] [PubMed]
- Zhang J, Khanna S, Jiang Q, et al. Efficacy of Anti-mesothelin Immunotoxin RG7787 plus Nab-Paclitaxel against Mesothelioma Patient-Derived Xenografts and Mesothelin as a Biomarker of Tumor Response. Clin Cancer Res 2017;23:1564-74. [Crossref] [PubMed]
- Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res 2006;12:7126-31. [Crossref] [PubMed]
- Szlosarek PW, Steele JP, Nolan L, et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol 2017;3:58-66. [Crossref] [PubMed]
- Beddowes E, Spicer J, Chan PY, et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers. J Clin Oncol 2017;35:1778-85. [PubMed]
- Ascierto PA, Scala S, Castello G, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol 2005;23:7660-8. [Crossref] [PubMed]
- Glazer ES, Piccirillo M, Albino V, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol 2010;28:2220-6. [Crossref] [PubMed]
- Izzo F, Marra P, Beneduce G, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 2004;22:1815-22. [Crossref] [PubMed]
- Yang TS, Lu SN, Chao Y, et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 2010;103:954-60. [Crossref] [PubMed]
- Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: Transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol 2017;8:37-53. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Linton A, van Zandwijk N, Reid G, et al. Inflammation in malignant mesothelioma - friend or foe? Ann Cardiothorac Surg 2012;1:516-22. [PubMed]
- Awad MM, Jones RE, Liu H, et al. Cytotoxic T Cells in PD-L1-Positive Malignant Pleural Mesotheliomas Are Counterbalanced by Distinct Immunosuppressive Factors. Cancer Immunol Res 2016;4:1038-48. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of Expression of Programmed Cell Death 1 Ligand 1 (PD-L1) in Malignant Pleural Mesothelioma (MPM). PloS One 2015;10:e0121071. [Crossref] [PubMed]
- Khanna S, Thomas A, Abate-Daga D, et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3+ T Cells Highly Expressing PD-L1 and the PD-L1+ Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti–PD-L1 Antibody Avelumab. J Thorac Oncol 2016;11:1993-2005. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, et al. B7-H1 Expression in Malignant Pleural Mesothelioma is Associated with Sarcomatoid Histology and Poor Prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Kindler H, L., Scherpereel A, Calabrò L, et al. Tremelimumab as second- or third-line treatment of unresectable malignant mesothelioma (MM): Results from the global, double-blind, placebo-controlled DETERMINE study. J Clin Oncol 2016;34:abstr 8502.
- Alley EW, Molife LR, Santoro A, et al. Abstract CT103: Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: Preliminary results from KEYNOTE-028. Cancer Res 2015;75:CT103. -CT. [Crossref]
- Quispel-Janssen J, Zago G, Schouten R, et al. OA13.01 A Phase II Study of Nivolumab in Malignant Pleural Mesothelioma (NivoMes): with Translational Research (TR) Biopies. J Thorac Oncol 2017;12:S292-S3. [Crossref]
- Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34:abstr 8503.


