Immunotherapy for malignant pleural mesothelioma: current status and future directions
Introduction
Epidemiology of thoracic malignancies
Malignant pleural mesothelioma (MPM) is a rare cancer of the pleura. The most commonly associated cause of MPM is asbestos exposure and, while the use of asbestos has been banned in most developed countries, it is still being used in many developing countries (1). Global incidence of MPM is likely underreported and, incidence in developed countries will likely peak in the second and third decades of the century (2). Individuals diagnosed with MPM have poor prognoses that are likely secondary to the difficulties encountered when establishing diagnosis and the advanced stage of the disease at time of diagnosis. Median survival is 12 months without treatment and, with treatment, the 6-month, 1-year, and 5-year overall survival (OS) rates are 55%, 33%, and 5%, respectively (3).
Currently established therapy
Systemic chemotherapy is a standard component in the treatment of patients with either resectable or unresectable disease. The phase III EMPHACIS trial (n=456) compared pemetrexed and cisplatin combination therapy with cisplatin alone and demonstrated a 3-month survival benefit (12.1 vs. 9.3 months) (4). Surgical resection for MPM is a controversial topic with no universally accepted guidelines. The most extensive surgical intervention for MPM is extrapleural pneumonectomy (EPP), which entails en bloc resection of the visceral and parietal pleura, pericardium, ipsilateral hemidiaphragm, and lung. The MARS trial compared patients who received EPP with those who did not and concluded that EPP offers no benefit and may actually harm patients; it is important to note that this study was a feasibility study that was conducted with a small cohort of patients (5). Alternatively, cytoreductive lung-sparing procedures, such as pleurectomy/decortication and extended pleurectomy/decortication, are associated with improved survival rates; however, they are also associated with considerably higher morbidity than supportive care (6).
Regardless of the surgical method employed, the goal of surgery is the removal of macroscopic disease (R1 resection). As such, adjuvant radiation therapy is used to control microscopic disease (7). Intensity-modulated pleural radiation therapy (IMPRINT) has been developed to target pleural surfaces directly while limiting lung exposure; it has been shown to be safe with no reported episodes of severe radiation pneumonitis (8).
Unfortunately, as a solitary therapy none of these modalities have been proven effective against MPM. Completion of trimodality therapy—which is the combination of chemotherapy, surgery, and radiation—can be difficult due to limited access to high volume centers, disease progression during therapy, and treatment side effects (9). The challenges associated with the delivery of trimodality therapy for MPM along with limited success underscores the need to develop new and efficacious therapies. Immunotherapy is one such emerging therapeutic modality that harnesses the power of the human immune system against cancer cells.
Immunoediting and immunotherapy
Effective immunosurveillance by the immune system protects an individual from the development and uncontrolled growth of malignancies (10). Immunoediting describes the process where an immunocompetent host can develop cancer and it consists of three phases—elimination, equilibrium, and escape (10). Elimination entails the activation of host immunity that results in tumor cell death. If unsuccessful, tumor cells may enter the equilibrium phase wherein tumor growth is maintained chronically. Alternatively, tumor cells can adapt via various mechanisms such as recruitment of regulatory cells, production of T-cell suppressant cytokines, and upregulation of co-inhibitory receptors by T cells. These adaptations result in tumor variants that can potentially overcome host immunity, thus entering the escape phase. At this point, clinically detectable lesions develop and the physical symptoms of cancer by the host are evident (10).
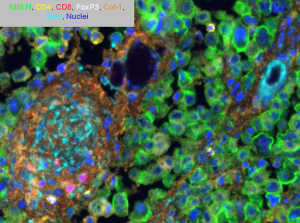
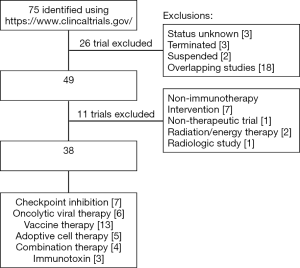
Our lab has been able to quantitatively characterize the composition of the immune microenvironment using immunohistochemistry and we have shown that the presence of inflammatory cells in the stroma or the relative proportions of specific immune cells are prognosticative (11,12). Using multiplexed immunofluorescence, in addition to quantification, we have been analyzing the localization and co-localization of immune cells. We are now able to explore their spatial relationship between tumor cells and the stroma on a single slide (Figure 1). In the ongoing battle between protumor and antitumor forces, a detailed understanding of the complex tumor immune microenvironment will be necessary to tilt the balance towards an antitumor immune response. In this review, we will provide a brief overview of the various immunotherapeutic strategies and discuss the results of clinical trials that are treating MPM (Figure 2).
Checkpoint blockade
One method of adaptation by tumor cells is the upregulation of cell surface inhibitory ligands. Tumor-infiltrating immune cells express inhibitory receptors that bind to these inhibitory ligands resulting in immune cell inhibition. These inhibitory receptors—also known as immune checkpoints—act as a regulatory system that prevent autoimmunity, but at the same time can play a crucial role in tumor development. Checkpoint blockade therapy utilizes antibodies to block this inhibitory signaling, thereby preventing inhibition of immune cells. Several different checkpoint inhibitors have been targeted to treat MPM, namely cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PD-L1) pathways.
CTLA-4 inhibition
CTLA-4 is a glycoprotein that is expressed by activated T cells and regulatory T cells (Tregs) (13). CTLA-4 competes with the costimulatory receptor CD-28 for B7 ligands (CD-80 and CD-86) expressed on antigen presenting cells (APCs). CTLA-4/CD-80 binding results in signaling that directly inhibits T-cell effector function (14).
The first clinical success of inhibiting this pathway was seen with ipilimumab (IgG1 antibody against CTLA-4), which demonstrated a significant improvement in OS of patients with unresectable stage III or IV melanoma (15). These results have led to multiple clinical trials assessing anti-CTLA-4 checkpoint inhibitors for the treatment of various solid malignancies including MPM.
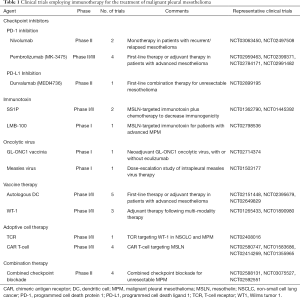
Tremelimumab was evaluated in a phase II clinical trial of 29 patients with unresectable MPM. Disease control was achieved in 52% of patients with a median duration of 10.9 months (95% CI, 8.2–13.6 months) (16). Four patients in this trial had at least one grade 3 or 4 adverse event. There is only one active clinical trial as the Phase II clinical trial that compared tremelimumab as a solitary therapy with placebo for patients with unresectable MPM is no longer recruiting patients (Table 1).
Full table
PD-1 inhibition
PD-1 is a fellow member of the B7-CD28 superfamily and another key immune checkpoint receptor. PD-1 is expressed by activated T-lymphocytes, B-lymphocytes, and natural killer (NK) cells. It binds to PD-L1 and PD-L2 ligands that are expressed on tumor cells and stromal cells (17). This interaction leads to decreased T-cell cytotoxicity, cytokine release, and proliferation, and ultimately results in T-cell exhaustion (18).
Nivolumab is a human IgG4 monoclonal antibody that targets PD-1. Its safety, antitumor activity, and pharmacokinetics were initially assessed in a Phase I trial of patients with advanced NSCLC (17). Its optimal dose was determined to be 3 mg/kg with an objective response rate (ORR) of 32%. Subsequently, multiple trials have tested nivolumab as a monotherapy or in combination with other therapies. Pembrolizumab is another human IgG4 antibody that targets PD-1 and was assessed in the KEYNOTE-001 trial. This trial demonstrated that patients with NSCLC who had >50% membrane expression of PD-L1 exhibited a higher response rate and exhibited longer OS and progression free survival (PFS) than patients with <50% PD-L1 expression (19). The follow-up KEYNOTE-028 trial studied response to pembrolizumab in PD-L1+ solid tumors; the patient cohort included 25 patients with MPM. Patients received 10 mg/kg every 2 weeks for 2 years or until progressive disease or severe toxicity was observed. The overall response rate was 20% (n=5) and 13 patients (52%) had stable disease, which resulted in a disease control rate of 72% (20).
Anti-PD-L1 inhibition
The KEYNOTE-010 and KEYNOTE-028 trials clearly demonstrated that PD-L1 plays a critical role in determining the efficacy of anti-PD-1 therapy. Furthermore, PD-L1 has been known to be upregulated in a variety of malignancies and its expression correlates with poor prognoses (21). Specifically, Mansfield et al. has demonstrated that PD-L1 expression occurred in approximately 40% of the 106 mesothelioma specimens and that higher expression was correlated with worse prognosis (5.0 vs. 14.5 months) (22). Additionally, patients with PD-L1+ tumors have been shown to have lower median survival (4.79 vs. 16.3 months, P=0.012) (23). As a result of these findings, several anti-PD-L1 therapies are currently being investigated in prospective clinical trials (Table 1).
The ongoing JAVELIN trial is assessing avelumab, a human anti-PD-L1 IgG1, in patients with metastatic or locally advanced solid tumors including 53 MPM patients. Early results have shown a disease control rate of 56.6% and median PFS of 17.1 weeks (95% CI, 6.1–30.1 weeks) (24).
Checkpoint blockade immunotherapy does have several limitations. Immune-related adverse events (irAE) are unique side effects/toxicities that occur as a result of stimulating the immune system. Common irAEs associated with checkpoint inhibitors are fatigue, rash, colitis, and hepatitis. Rarer and more severe side effects include endocrinopathies such as hypophysitis and pneumonitis (25). Unfortunately, biomarkers predicting safety or predilection for irAEs are lacking. Similarly lacking are methods of identifying which patient population will benefit most from checkpoint inhibition. Finally, blocking of checkpoint inhibition pathways has been shown to lead to an upregulation of different inhibitory checkpoints, such as TIM-3 and LAG-3 (26), which is an obstacle that will receive increased attention going forward.
Immunotoxin
Immunotoxin immunotherapy utilizes an antibody or antibody fragment fused to potent toxins—including bacterial or plant-derived ribosomal proteins—to attack tumor cells. Once the antibody binds to its targeted antigen, the toxin is chaperoned to the cytosol of a cancer cell via endocytosis and results in cell death. Immunotoxins have shown promising results in hematologic malignancies, specifically hairy cell leukemia, wherein a majority of patients achieved complete remission after therapy (27). Although immunotoxins have been used to treat MPM, its application in other solid malignancies generally has been limited.
Mesothelin (MSLN) is a cell surface protein that is overexpressed in a variety of solid tumors including mesothelioma, ovarian, and pancreatic cancers. SS1P is an immunotoxin consisting of an anti-MSLN antibody fragment fused to Pseudomonas exotoxin A. Hassan et al. initially demonstrated that SS1P can be administered safely with moderate antitumor efficacy as a monotherapy (28); however, when it was combined with pemetrexed and cisplatin there was an impressive antitumor response (29). In a cohort of 13 patients with MPM, 77% of patients demonstrated at least a partial response. A recently completed Phase I clinical trial that tested SS1P combined with cisplatin and pemetrexed is awaiting data analysis. LMB-100 is another MSLN-targeted immunotoxin that is currently being assessed in an open and recruiting Phase I clinical trial.
One limitation of immunotoxin therapy is the development of patient-derived neutralizing antibodies against the toxin (29). Immunosuppressants can be used to counteract the development of these antibodies but they are associated with multiple side effects as well. Additionally, since there are few target antigens identification of additional targets is of great importance going forward.
Oncolytic viral therapy
Oncolytic viruses are replicating viruses that harness the replicative life cycle of the virus by preferentially infecting and lysing tumor cells, and then releasing viral progeny, which results in death of neighboring cancer cells (30). The concept of viruses exhibiting antitumor activity has been described since the early 1900s when patients with leukemia and lymphoma had undergone spontaneous remission after various viral infections (31). To date, there have been several clinical trials that have investigated oncolytic viral therapy for solid tumors including MPM.
Granulocyte-macrophage colony stimulating factor (GMCSF) can facilitate antitumor response through recruitment of CD8+ T cells and NK cells. Attempts at systemic administration of GMCSF have been limited by poor tumor penetration and toxic side effects (32). To minimize these side effects and increase the concentration of GMCSF in the tumor microenvironment, oncolytic viruses that express GMCSF have been employed. ONCOS-102 is an attenuated adenovirus modified to express GMCSF. Ranki et al. demonstrated in a phase I clinical trial that ONCOS-102 is safe and well tolerated. Additionally, they showed an increase in pro-inflammatory cytokines, tumor infiltrating lymphocytes (TILs), and inhibitory ligands (e.g., PD-L1) in 11 out of 12 patients. These findings suggest that ONCOS-102 can be used as a priming agent that is implemented with other immunotherapies (33). As such, a phase Ib/II clinical trial is currently recruiting patients to assess the safety of ONCOS-102 combined with pemetrexed/cisplatin; afterward, a randomized cohort will be assessed to determine if there is a correlation between clinical outcomes and immunologic data (Table 1). Additionally, there is an open and recruiting trial assessing combination ONCOS-102 with durvalumab—a checkpoint inhibitor—for patients with peritoneal malignancies including peritoneal mesothelioma. These trials may pave the way for a clinical trial assessing combination therapy for MPM.
Vaccinia is an enveloped virus that is highly immunogenic but safe when administered to patients, as evidenced by the fact that it was the active component of the vaccine that eradicated smallpox. This virus has a large genome that allows for insertion of multiple genes without compromising its ability to replicate (34). Vaccinia was also effective at killing MPM cell lines in vitro and in an orthotopic mouse model of MPM; tumor burden was significantly reduced with improved 30-day survival (35). These preclinical results led to a clinical trial where the GL-ONC1 vaccinia virus was administered intrapleurally to patients with malignant pleural effusions; this trial has been completed at our center and data analysis is pending. There is another open and recruiting clinical trial treating patients with solid organ tumors, including MPM, which combines GL-ONC1 and eculizumab, a monoclonal antibody that targets the complement cascade (Table 1).
Attenuated measles virus (MV) is similar to vaccinia and has been proven safe when administered to patients as a vaccine. The first clinical trial employing oncolytic MV was a small, open-label Swiss study of patients with cutaneous T-cell lymphoma. The MV was injected intratumorally and, after 28 days, 5 of 6 tumors had regression and 1 of those 5 resolved completely. Additionally, oncolytic MV exhibited an abscopal effect, which means that after injection into the primary tumor there was regression of metastatic tumor sites (36). These promising results have led to the application of oncolytic MV for various solid tumors including ovarian cancer, squamous cell head and neck malignancies, and MPM. These trials include an open and recruiting Phase I clinical trial investigating intrapleural delivery of MV therapy for patients with stage I-IV or recurrent MPM.
Although this strategy of immunotherapy has been slow to develop in the clinic, there are several reasons to believe this will be an area of growth going forward for the treatment of MPM and other solid tumors. Viruses are structurally diverse and provide a multitude of delivery options. Additionally, oncolytic viruses can potentiate antitumor immunity while causing minimal toxicity in patients. Nonetheless, there are several limitations to oncolytic viral therapy. Systemic delivery of therapy can elicit the development of neutralizing antibodies that result in sequestration by Kupffer cells in the liver and spleen, thus resulting in limited intratumoral virus spread (37).
Anticancer vaccines
Cancer vaccination is a method of immunotherapy that involves activating an immune response using a specific peptide antigen with the purpose of inducing specific antitumor immunity against tumor-associated antigens. Antigen-exposed autologous dendritic cells (DC) are perhaps the most potent APC (38). They are capable of capturing and processing tumor antigens, expressing co-stimulatory molecules, and secreting cytokines to initiate immune responses. As such, DCs have been used increasingly in tumor cell vaccinations for MPM. When combined with cyclophosphamide, 7 of 10 patients with MPM survived ≥24 months and there were 2 patients who survived 50 and 66 months after treatment (39). There are several open and recruiting clinical trials of DC-VAC for the treatment of MPM (Table 1). Additionally, there are open and recruiting clinical trials evaluating targeted vaccination as a monotherapy or combined with chemotherapy.
In order for cancer vaccinations to be a successful treatment for solid tumor malignancies there are two limitations that must be overcome—the lack of strong expression of target antigens on cancer cells and the reliance on the ability of the host immune system to mount an immune response.
Adoptive cell therapy (ACT)
ACT entails the collection of immune cells from peripheral blood or the tumor itself, followed by isolation, modification, and ex vivo expansion of the targeted immune cells. The modified immune cells are then reinfused back to the patient as therapy (40). ACT offers the advantage of targeting effector cells to a specific tumor-associated antigen (TAA) and leads to direct cytotoxicity.
ACT was first used as a treatment modality in the 1960s but has seen rapid growth in development and application since the turn of the century. The first application of adoptive T-cell therapy involved in vitro activation, expansion, and reinfusion of antigen-specific TILs isolated from fresh cancer specimens (41). TIL therapy initially showed success in the treatment of malignant melanoma but its application to other malignancies has presented several challenges. The extraction of sparse tumor-reactive lymphocytes and subsequent isolation and expansion of T cells that retain specificity and functionality can be difficult (42). Additionally, prolonged clinical response to TIL therapy requires lymphodepletion (40). Given these difficulties, there have been several different strategies that aim to harness the antitumor efficacy of T cells including genetically modifying the T-cell receptor (TCR) and chimeric antigen receptor (CAR) modified T cells.
TCR therapy
TCR therapy consists of genetically engineering large populations of T cells to target specific TAAs. T-cell specificity is mediated through the α- and β-chain heterodimers of the TCR complex (43). The α/β chain gene can be modified to target a specific antigen and then transduced retrovirally into T cells from healthy donors (43). TCR therapy is major histocompatibility complex (MHC)-dependent. This means that in order for T-cell activation to occur the TCR must bind to a specific MHC-antigen complex. Because of this, TCR therapy is restricted to MHC-matched patients (41). Another limitation of TCR therapy is that tumors can escape the cytotoxicity of TCRs by downregulating MHC class I expression (43). There is currently an open and recruiting clinical trial involving TCR immunotherapy targeting WT-1 (Table 1).
Chimeric antigen receptor T-cell therapy
The limitations of TCR therapy have led to the development of CAR-modified T cells that are not restricted by MHC, which means that CAR-modified T cells can potentially target any antigen (i.e., any tumor). A CAR construct consists of an extracellular antigen-binding domain that is hinged to one or more intracellular signaling domains (42). Once constructed, the CAR is then transduced into autologous T cells and transfused back to the patient as therapy. CARs specific for CD19, a B-cell activation receptor, have been successful in the treatment of B-cell malignancies including acute lymphoblastic leukemia and chronic lymphocytic leukemia (44). These successes have led to rapid growth in this intriguing subfield of immunotherapy.
Our laboratory work is focused on investigating CAR T-cell therapy for MSLN-expressing tumors. As previously mentioned, MSLN is expressed in a variety of malignancies including MPM. Our lab has demonstrated that MSLN is uniformly and strongly expressed on MPM cells and has limited expression on normal tissue (45). We established an orthotopic mouse model of MPM and demonstrated that regional delivery of MSLN-targeted CAR T cells were able to eradicate tumors at a 30-fold lower dose than systemically delivered CAR T cells (46). These intrapleurally administered CAR T cells outperformed the systemically delivered CAR T cells in terms of T-cell activation, proliferation, persistence, tumor eradication, and survival. The underlying biologic reasons for the observed benefits following regional delivery are the avoidance of pulmonary sequestration that occurs following systemic delivery, whereas intrapleural delivery results in immediate antigen-activation and CD4-dependent additive benefits. These preclinical results have led to the development of an open and recruiting Phase I study where patients with MPM or other secondary pleural malignancies receive a single dose of intrapleurally delivered MSLN-targeted CAR T cells, with or without prior cyclophosphamide therapy (Table 1). MSLN-targeted CAR T-cell therapy trials are also being conducted at the National Cancer Institute and the University of Pennsylvania.
Combination therapy
The impressive, yet limited successes, seen with the administration of single immunotherapeutic agents have led to a variety of combination strategies. The CheckMate 067 trial compared dual checkpoint blockade therapy (nivolumab and ipilimumab) with monotherapy for patients with advanced stage melanoma. Combination therapy resulted in an ORR of 60% and a median PFS of 11.5 months compared with 6.9 and 2.9 months for nivolumab or ipilimumab alone, respectively (47).
These promising results have led to clinical trials that combine different methods of immunotherapy. There are currently two open and recruiting clinical trials evaluating the efficacy of combination tremelimumab and durvalumab (anti-PD-L1) (Table 1). There have been preclinical studies that have assessed a combination of checkpoint inhibitors with oncolytic viruses as well as antitumor vaccinations; however, these preclinical studies have yet to be translated into clinical trials. The limitations of checkpoint inhibition monotherapy—including lack of prognosticative biomarkers and irAEs—apply to combination therapy as well and will undoubtedly be limitations to future clinical successes.
Our group recently has demonstrated that a combination of CAR T-cell therapy and checkpoint blockade results in remarkable tumor regression and improved OS in mice with MPM (48); however, repeated administration of PD-1 blocking antibodies were required to achieve these results. In an effort to circumvent repeated dosing, we have constructed CAR T cells that express a T-cell intrinsic PD-1 dominant negative receptor (DNR) that binds to PD-L1 but does not transmit inhibitory signaling to the T-cell (48). Such innovative techniques can potentially circumvent the toxicities associated with combination CAR T-cell therapy and checkpoint blockade.
Conclusions
Immunotherapy employs novel therapies and is a promising and rapidly developing approach for treatment of solid tumor malignancies. This review highlights some of the successes as well as the limitations of immunotherapy for MPM. The barriers to success that must be overcome are identification of biomarkers that are capable of determining which patients will benefit most from specific treatment options, identification of target antigens, and overcoming the mechanisms of resistance and inhibition within the complex tumor immune microenvironment. There are many early phase clinical trials that have assessed immunotherapy in different lines of therapy as monotherapy and in combination with other treatment modalities. The plethora of results from these diverse trials, both the failures and successes, have laid the foundation for the future of this exciting therapeutic approach.
Acknowledgements
We thank Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
Funding: The author’s laboratory work is supported by the U.S. Department of Defense (BC132124 and LC160212 to PS Adusumilli); the National Institutes of Health (P30 CA00874 and R21 CA164568-01A1 to PS Adusumilli); the Mesothelioma Applied Research Foundation; the Baker Street Foundation; the Derfner Foundation; the Joanne and John DallePezze Foundation; and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8. [Crossref] [PubMed]
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491-6. [PubMed]
- Milano MT, Zhang H. Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol 2010;5:1841-8. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Zahid I, Sharif S, Routledge T. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2011;12:812-7. [Crossref] [PubMed]
- Kotova S, Wong RM, Cameron RB. New and emerging therapeutic options for malignant pleural mesothelioma: review of early clinical trials. Cancer Manag Res 2015;7:51-63. [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Suzuki K, Kadota K, Adusumilli PS. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011;60:1721-8. [Crossref] [PubMed]
- Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015;4:e1009285. [Crossref] [PubMed]
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002;168:4272-6. [Crossref] [PubMed]
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013;13:5. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med 2012;366:2443. [Crossref] [PubMed]
- Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol 2010;7:389-95. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. OncoTargets Therapy 2014;7:567-73. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Zugazagoitia J, et al. Analyisis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. [Crossref] [PubMed]
- Hassan R, Thomas A, Patel M, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34:abstr8503.
- Kumar V, Chaudhary N, Garg M, et al. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol 2017;8:49. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Kreitman RJ, Wilson WH, Bergeron K, FitzGerald DJ, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 2001;345:241-7. [Crossref] [PubMed]
- Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144-9. [Crossref] [PubMed]
- Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. [Crossref] [PubMed]
- Adusumilli PS, Chan MK, Chun YS, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther 2006;5:48-53. [Crossref] [PubMed]
- Ahmad Z, Kratzke RA. Novel oncolytic viral therapies in patients with thoracic malignancies. Oncolytic Virother 2016;6:1-9. [Crossref] [PubMed]
- Arellano M, Lonial S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics 2008;2:13-27. [PubMed]
- Ranki T, Pesonen S, Hemminki A, et al. Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer 2016;4:17. [Crossref] [PubMed]
- Kelly KJ, Woo Y, Brader P, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther 2008;19:774-82. [Crossref] [PubMed]
- Belin LJ, Ady JW, Lewis C, et al. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery 2013;154:486-95. [Crossref] [PubMed]
- Aref S, Bailey K, Fielding A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016;8:E294. [Crossref] [PubMed]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012;30:658-70. [Crossref] [PubMed]
- Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012;13:e301-10. [Crossref] [PubMed]
- Cornelissen R, Hegmans JP, Maat AP, et al. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31. [Crossref] [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
- Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol 2010;2010:956304.
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388-98. [Crossref] [PubMed]
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003;3:35-45. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012;18:2478-89. [Crossref] [PubMed]
- Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. [Crossref] [PubMed]


