BAP1, a tumor suppressor gene driving malignant mesothelioma
Introduction
Malignant mesothelioma (MM) is a relatively rare cancer frequently linked to prior exposure to asbestos. Approximately 3,000 new cases of MM are diagnosed annually in the United States. As a cancer of the mesothelial cell lining that surrounds various organs, the majority of MM (~80%) occurs in the pleural cavity surrounding the lungs, whereas most of the remaining (peritoneal) tumors arise in the abdominal cavity. Prognosis is generally very poor with a median survival of ~9–12 months for pleural cases (1,2). Similar to other cancers, MM is a disease that can result from the interactions between environmental carcinogenic factors (e.g., asbestos) and genetic predisposing factors, only one of which has been identified to date. At the somatic genetic level, losses of chromosome regions 3p21.1, 9p21.1, and 22q12.2 are frequently observed in MM. The critical driver genes located at 9p21.1 and 22q12.2 were first reported more than 2 decades ago as being the tumor suppressor loci CDKN2A and NF2, respectively (3,4). Only relatively recently was the BAP1 gene determined to be the driver gene at 3p21.1 that is frequently somatically inactivated (5), and germline mutations of BAP1 predisposing to MM were reported independently at about the same time (6). Numerous studies have confirmed that BAP1 is a major MM susceptibility gene (see below), and that most MMs (>80%) harbor somatic alterations of the CDKN2A locus, which encodes the tumor suppressor proteins p16INK4A and p14ARF (regulators of the critical Rb and p53 pathways, respectively), 60% harbor somatic mutations and exonic deletions of the BAP1 gene and 30–50% show inactivation of NF2.
Somatic and germline mutations of BAP1
A seminal paper by Jensen et al. described the localization of the BAP1 gene to chromosomal region 3p21.2–p21.31, a region that is frequently deleted in lung carcinomas and many other cancers (7). The group identified somatic BAP1 deletions/mutations in two non-small cell lung cancer and one small cell lung cancer cell lines. Although several noteworthy functional studies would ensue between 2008 and 2010 (8-12), more than a decade would pass before the seminal genomic report of frequent (~85%) involvement of somatic BAP1 mutations in metastatic uveal melanomas (UM) (13). These ocular tumors are categorized as either class 1 (low metastatic potential) or class 2 (high metastatic potential tumors), the latter strongly correlated with monosomy 3. Utilizing exome and Sanger sequencing technology, somatic BAP1 mutations were identified in 26 of 31 (84%) class 2 primary UMs but in only 1 of 26 class 1 primary tumors, thus implicating BAP1 mutations in UM metastatic capacity. Of particular interest to us was the discovery of a germline BAP1 mutation in one of the patients with a class 2 UM, though no extended family history studies were performed (13). Somatic BAP1 mutations in MMs were first reported in 2011 by Bott et al. (5). They used a candidate gene sequencing approach to scrutinize tumors for mutations in genes located in chromosomal region 3p21 and discovered that BAP1 was mutated in 12 of 53 (23%) MMs.
At about the same time, the first description of germline BAP1 mutations being associated with familial MM was reported (6). We described two U.S. families with a high incidence of MM and other types of cancers, such as renal carcinoma; additionally, two UMs were seen in one family. A germline splice site mutation in BAP1 intron 6 in DNA from one family (W family) was shown to lead to aberrant skipping of exon 7 during mRNA processing and a predicted protein truncation. A nonsense mutation in exon 16 was present in the second (L) family, also leading to a predicted protein truncation. Published simultaneously in the same issue of Nature Genetics, another report described germline mutations of BAP1 in two families with atypical melanocytic tumors, UMs, and cutaneous melanomas (CMs) (14). In a subsequent report published later in 2011, a third independent study reported results of a mutation screen of 53 unrelated UM patients with known high risk for hereditary cancer, and notably, a single patient was identified with a germline BAP1 truncating mutation that was associated with UM and multiple other cancers in this patient’s family (15). Biallelic inactivation of BAP1 and decreased BAP1 expression were identified in the UM, lung adenocarcinoma and meningioma from three family members who were mutation carriers. Notably, other cancers observed in this family include MM, CM, and meningioma. Since then, numerous reports have expanded on the discovery of germline mutations in families or individuals with these and other cancers [BAP1 tumor predisposition syndrome (TPDS)] (16-29). In the OMIM (Online Mendelian Inheritance in Man) database, the disorder is now referred to as TPDS #614327 (http://www.omim.org/entry/614327?search=bap1&highlight=bap1), which is inherited in an autosomal dominant manner with individuals carrying a heterozygous BAP1 mutation being at high-risk for various tumors, including benign melanocytic tumors (atypical Spitz tumors) and multiple malignant tumors, such as UM, CM, “MM on exposure to asbestos”, and other cancer types (i.e., lung adenocarcinoma, meningioma, and renal cell carcinoma). A list of the tumors that are associated with the BAP1 TPDS was recently summarized by Pilarski et al., 2016; (https://www.ncbi.nlm.nih.gov/books/NBK390611/) (30). Confirmed BAP1-TPDS tumors include the following: atypical Spitz tumors, UM, MM, CM, clear cell renal cell carcinoma, and basal cell carcinoma. Unconfirmed neoplasms (with conflicting evidence regarding inclusion in the syndrome) include the following (in alphabetic order): breast cancer, cholangiocarcinoma, meningioma, neuroendocrine tumors, non-small cell lung adenocarcinoma, and thyroid cancer.
Unique MM clinical characteristics associated with BAP1 mutations
A comprehensive analysis of MM high-risk families has led to the discovery of some interesting differences in the clinical features of MM patients with or without germline BAP1 mutations (16). We examined the germline BAP1 mutation status of 150 MM patients with a family history of cancer, 50 asbestos-exposed control individuals with a family history of cancers other than MM, and 153 asbestos-exposed control individuals without familial cancer. No BAP1 mutations were identified in the control cohorts, but were identified in 9 of 150 (6%) individuals with a family history of cancer. Firstly, the median age of MM diagnosis was significantly younger among the 9 BAP1 mutation carriers as compared to non-carriers (58 vs. 68 years). This earlier age of tumor onset is similar to that observed in other cancer predisposition syndromes (31), such as Hereditary Breast and Ovarian Cancer Syndrome (32) and Li-Fraumeni Syndrome (33). Secondly, there was an overrepresentation of peritoneal MMs (5 of 9) and a tendency for epithelioid MM among the mutation carriers compared to non-carriers (16). Finally, but very remarkably, these 9 MM individuals have a better overall survival after MM diagnosis (60 vs. 17 months among the non-carriers) (16), which is similar to survival data findings from another group of investigators (34). This notable clinical characteristic is likely due to the mutation carriers being younger and predominantly having peritoneal, epithelioid disease, which has an overall better prognosis than pleural and sarcomatoid MM, respectively (1,2). Collectively, these findings suggest that MM patients presenting with a family history of cancer should be considered for BAP1 mutation screening to identify carriers who might benefit from routine monitoring for the purpose of early detection and intervention.
Sanger sequencing has revealed somatic BAP1 point mutations in 20–25% of sporadic MM samples (5,6,35-37). Subsequent studies using next-generation and multiplex ligation-dependent probe amplification (MLPA) platforms have revealed a significantly higher incidence (~60%) of BAP1 alterations in MMs from both BAP1-TPDS individuals, sporadic cases and cell lines, with most of the additional alterations consisting of deletions of one or more BAP1 exons, which was not detectable by Sanger sequencing (5,6,35-37). MMs with either point mutations or deletions were each found to exhibit loss of nuclear BAP1 staining due to protein truncation and loss of the BAP1 carboxyl-terminal nuclear localization signal (NLS) (38). Tumors harboring somatic BAP1 alterations were also found to possess unique pathological and clinical characteristics. Thus, BAP1 immunohistochemical analysis of 123 MM samples indicated that high BAP1 expression (indicative of no mutations) correlated with a shorter survival time (39), which is similar to findings from studies of MM patients with germline BAP1 mutations (16,34). Also comparable, was the association of somatically inactivated BAP1 with the epithelioid MM subtype (36). Since BAP1 can influence the regulation of gene expression epigenetically or through deubiquitination of transcription factors (see below), it is plausible that these different patterns of gene expression can lead to variations in MM tumor subtypes (35,40,41).
Similar to Li-Fraumeni syndrome (33), there are a number of reported BAP1 mutation families where individuals are afflicted with more than one type of primary tumor (23,42-44), strongly suggesting multiple tissue lineages being targeted by BAP1 deficiency. Cancer incidence data from published papers reporting germline BAP1 mutations indicated that the two most common primary tumors observed together are MM and UM (7 cases), melanocytic tumors and CM (7 patients), CM and basal cell carcinoma (7 patients), and MM and CM (6 cases) (Table 1).
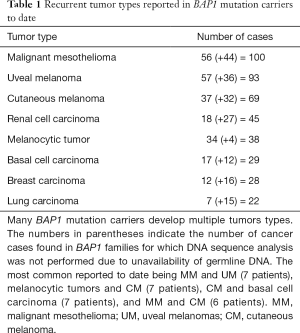
Full table
BAP1 gene, protein structure, and function
Rauscher, Prendergast and colleagues were the first to clone and characterize the BAP1 gene (7). BAP1 was discovered through a yeast two-hybrid screening for BRCA1 ring finger domain interacting proteins. Cloning of the full-length BAP1 cDNA and analysis of the predicted protein product indicated that gene encodes a 729-amino acid protein with a molecular weight of 81 kDa, although subsequent immunoblot studies revealed a protein 90 kDa in size, likely due to post-translational modifications. The amino terminus of the BAP1 protein has amino acid homology with a class of thiol proteases, designated Ubiquitin C-Terminus Hydrolases (UCH). A BARD1 binding site and HCF-1 (host cell factor 1) binding domain are located at the amino terminus and middle of the BAP1 protein, respectively. The C-terminal region encompasses protein interacting domains for YY1 (Ying Yang 1), BRCA1 and ASXL1/2. A NLS is positioned near the very end of the protein at residues 717 to 722. Similar to the better-known post-translational modifications through phosphorylation, ubiquitination can play a role in modifying proteins to cause changes in cellular signaling. As a nuclear localized, deubiquitinating enzyme, the BAP1 protein complexes with ASXL1/2 to form the Polycomb repressive group deubiquitinase Complex (PR-DUB) that functions in epigenetic regulation of gene expression through chromatin structure modifications (11,45). The PR-DUB complex functions in the deubiquitination of monoubiquitinated histone H2A K119 to promote gene expression via chromatin relaxation. The PR-DUB works in opposition to the Polycomb Repressive Complex 1 (PRC1) that catalyzes the monoubiquitination of H2A, which plays a physiological role in stem cell pluripotency, differentiation, and embryonic development (11,45).
A role for BAP1 in cell proliferation has been suggested from studies showing BAP1’s ability to deubiquitinate the HCF-1 transcriptional cofactor (8,9,12). HCF-1 normally associates with E2F1, E2F3, and E2F4 transcription factors to help recruit repressors and activators to promote cell cycle progression at different stages. BAP1 was shown to deubiquitinate HCF-1 leading to a modest increase in HCF-1 protein levels (8,9). BAP1 and HCF-1 were also shown to be recruited to promoters to control gene regulation through the ability of the two proteins to bind to the YY1 transcription factor (12). Gene pathway analysis indicated that this interaction appears to control a number of different processes including cell cycle progression, cell survival, and metabolism (12).
A functional RNAi screen identified BAP1 as being a central player for efficient BRCA1 and RAD51 recruitment to ionizing radiation (IR)-induced foci in DT40 cells, a chicken B cell lymphoma cell line (46). Further studies using Bap1 knockout DT40 cells demonstrated that the cells had decreased survival after DNA damaging IR treatment. Accordingly, increased chromosomal instability was also observed in BAP1 KO cells, as indicated by chromosomal breakage. Furthermore, ChIP (chromatin immunoprecipitation) analysis demonstrated that BAP1 proteins are located at regions of double strand DNA breakage (DSB) (46). An independent study by Ismail et al. similarly revealed that BAP1 protein was co-recruited with phosphorylated histone H2AX (γ-H2AX; a marker for DSB) after human U2OS cells were laser microirradiated (47). ChIP assays also showed that BAP1 and γ-H2AX were recruited to sites of endonuclease-induced DSB (47).
The BAP1 protein was recently demonstrated to play an important role in inhibiting apoptosis caused by metabolic stress such as glucose deprivation (48). The unfolded protein response (UPR) protects cells from stress caused by misfolded proteins in the endoplasmic reticulum (i.e., glucose deprivation), and if the stress is unresolved, this leads to induction of apoptosis by depleting ATP and generating reactive oxygen species (ROS). The investigators demonstrated that under metabolic stress, BAP1 complexes with PRC1 to promote the expression of genes essential for UPR by directly binding to the genes’ promoters (48). Studies performed with several BAP1-null lung and renal cancer cell lines showed increased apoptotic induction following glucose deprivation, suggesting that the increased survival reported in patients with BAP1-negative MM may be due to the inability of these cancer cells to actively proliferate due to the stress caused by the high demand for glucose (48). Accordingly, it would be important in future work to test if BAP1-null MM tumor cells are more sensitive to ROS-promoting therapeutics.
Lessons from mouse models
The use of Bap1 genetically engineered mouse models provided undeniable evidence for BAP1 being an important tumor suppressor gene. Utilizing zinc-finger mediated genomic DNA modifications, we created three Bap1 mouse models (49,50). In the first study (50), a heterozygous Bap1-null model in the FVB mouse strain was created by introducing a deletion of exons 6 and 7. Similar to a previous study (51), homozygous mice were found to be embryonic lethal, indicating an essential embryonic function for the gene. More importantly, was the discovery that heterozygous Bap1 knock out (KO) mice were more susceptible to MM development after peritoneal injection of crocidolite asbestos (50). Compared to wild type (WT) littermates, there was a greater than 2-fold increased incidence of MM tumors in Bap1 KO mice (32% versus 72%, P<0.01) as well as an overall decrease in survival after asbestos exposure (55 versus 43 weeks median, P<0.0001). Moreover, MM tumors from KO mice were more aggressive than the tumors from WT mice. Greater staining for Ki-67, larger tumor sizes, and increased metastasis to the pancreas, liver, and intestine were observed in the tumors from KO mice. Moreover, RT-PCR analysis of MM cell lines derived from ascites demonstrated loss of expression of p16Ink4a, p19Arf, and p15Ink4b genes in MM cells derived from three WT mice but not in MM cells from two KO mice. Notably, however, normal mesothelial cells and MM cells from Bap1 KO mice showed downregulation of Rb through a p16Ink4a-independent mechanism, suggesting that predisposition of Bap1 KO mice to MM may be facilitated, in part, by cooperation between Bap1 and Rb. Finally, PCR analysis of genomic DNA indicated loss of the WT copy of the Bap1 gene from the MM cells of two KO mice but not in the MM cells of WT mice, consistent with Bap1 being a cancer predisposition gene (50). These unbiased genetic findings in an experimental model suggest that humans carrying a germline BAP1 mutation may likewise be predisposed to the carcinogenic effects of asbestos fibers.
Similar phenotypic differences were observed between WT mice and two other Bap1 mutant mouse models in FVB background that harbor knock-in mutations analogous to the germline L and W mutations reported in humans (49). The median survival of asbestos injected W and L mice was significantly shorter than in WT mice (48 and 46 vs. 60 weeks, respectively). Moreover, the incidence of MM development was at least 2-fold higher among the Bap1 knock-in mice compared to WT mice (74% and 71% vs. 35%, respectively). Interestingly, combining the data from all three mouse models (KO, L, and W) revealed that about two-thirds of the Bap1 mutant mice developed spontaneous tumors, with the majority of the tumors comprising ovarian sex cord stromal tumors (SCSTs) (49). Virtually all female mice developed SCST (some bilaterally) within 12–30 months of age. Array CGH and immunoblot analysis showed loss of the wild type copy of Bap1 in three SCST tested, indicating the importance of the Bap1 gene in SCST tumorigenesis. MMs were found in one KO (pleural and biphasic) and one W mouse (peritoneal and biphasic) that were not exposed to asbestos, i.e., spontaneous MMs. While no MMs were seen in a cohort of WT littermates, the difference in the incidence of MM between WT and Bap1-mutant mice was not statistically significant. Based on the very high incidence of MM formation in asbestos injected Bap1-mutant mice, there thus appears to be a very strong role for gene-environment interaction (i.e., exposure to asbestos) in MM development. A study published the following year provided further support for the increased susceptibility of Bap1 KO mice to asbestos-induced MM, including upon exposure to relatively low doses of these carcinogenic fibers (52).
Additionally, a study by a separate group of investigators demonstrated increased H3K27me3 levels in bone marrow cells from Cre-induced homozygous Bap1 KO mice (53). The tri-methyl modification of histone H3 has been previously shown to be accomplished by the EZH2 subunit of the PRC-2 complex. Other experiments showed that BAP1 mutant human MM cell lines were more susceptible to shRNA and small molecule targeting of EZH2, suggesting a novel therapeutic approach for BAP1-mutant malignancies (53). Interestingly, the use of BAP1 and EZH2 staining was proposed as a diagnostic tool to differentiate epithelioid/biphasic MM from benign mesothelial lesions in humans (54). The researchers reported combined loss of BAP1 staining and high EZH2 staining in the majority of MM specimens examined, but not in any benign lesions (54).
Quantitative proteomic analysis of tissues from inducible Bap1 KO mice revealed a role in metabolic homeostasis in the pancreas and liver (55). Elevated cholesterol biosynthesis but reduced expression of gluconeogenic and lipid homeostasis proteins were observed in the liver. In the pancreas, expression of pancreatitis protein markers was increased whereas expression of mitochondria proteins was decreased. These mice also exhibited hypercholesterolemia, hypoglycemia, lipid reduction in the liver, and pancreatic acinar cell degeneration (55). How such metabolic dysregulation is related to cancer predisposition or tumorigenesis is yet to be determined. However, it is known that increased cholesterol and lipid synthesis is required in cancer progression due to the high demand for both membrane lipids as well as for cell signaling [reviewed in (56,57)]. As a consequence, it may be possible to identify possible molecular targets for therapeutic and/or preventative regimens in MM patients and BAP1 mutation carriers, respectively. For example, Hedgehog inhibitors, such as GDC-0449 already available for basal cell carcinoma treatment, can be evaluated for efficacy in BAP1 deficient MM cells (57).
Preventative and therapeutic strategies for MM
Since MM, CM, and UM are universally lethal diseases, the best approach would be to prevent the development of these cancers in BAP1 mutation carriers through appropriate proactive measures. It has been suggested that these individuals avoid asbestos and smoking to decrease the possibility of developing MM (30). Furthermore, arc welding and excessive sun exposure should be avoided to decrease the likelihood of developing UM and CM, respectively. BAP1 mutation carriers are at risk of developing additional primary tumors during their lifetime. Thus, cancer survivors should have regularly scheduled dermatologic, ophthalmologic, pulmonary and renal evaluations to enhance the possibility of early detection and timely intervention.
Due to the important role BAP1 has with BRCA1 in homologous recombination repair (46), poly (ADP ribose) polymerase (PARP) inhibitors were tested for their efficacy in BAP1-null tumor cells. In one report, no differential sensitivity was observed between BAP1 WT and BAP1-mutant MM cells with the MK4827 (Merck) inhibitor (5). In another study using chicken DT40 cells (46), increased sensitivity to the PARP inhibitor, Olaparib, was observed in homozygous BAP1-null cells as compared to WT and heterozygous BAP1-null cells. A recent study implicated the importance of the levels of an alternative splice variant of BAP1 in conferring sensitivity to PARP inhibition (58). This alternative splice isoform leads to the loss of 12 amino acids within the catalytic and BARD1 binding domains. Transfection of BAP1-deficient ZL55 MM cells with this BAP1 isoform resulted in a 2- to 3-fold increased sensitivity to Olaparib compared to cells transfected with the full length BAP1 construct (58). Additional tests were carried out to determine if phosphoinositide 3-kinase (PI3K) inhibition, which can deplete BRCA1 protein levels, could have an additive effect with PARP inhibition. MM cells wild type for BAP1 were separated into two groups based on the ratio of the expression of the BAP1 splice isoform to that of the full-length isoform. Cells with higher splice isoform expression showed greater cell viability inhibition after combination treatment with Olaparib and GDC0980, a dual PI3K-mTOR inhibitor (58).
A histone deacetylase (HDAC) inhibitor, vorinostat, was previously evaluated in a phase 3 clinical trial involving MM patients who previously progressed after chemotherapy (59). Unfortunately, no improvements in overall survival resulted from vorinostat treatment (59). A recent study of MM cells and HDAC expression may have provided a reason for this disappointing clinical study result (60). The authors demonstrated that BAP1 can transcriptionally promote the expression of HDAC2. BAP1 knockdown led to a decrease in HDAC2 but an increase in HDAC1 expression (60). Interestingly, this altered HDAC imbalance led to an increase in MM cell sensitivity to vorinostat and other HDAC inhibitors. Thus, clinical trials of MM with HDAC inhibitors might prove more successful if recruitment of patients were to take into account the BAP1 mutation status of individual tumors.
As mentioned previously, EZH2 was proposed as a possible therapeutic target in MM tumors with BAP1 mutations (53). A clinical trial using an EZH2 inhibitor is already underway to test efficacy in human MM patients (ClinicalTrials.gov Identifier: NCT02860286). This Phase 2 trial is recruiting patients with relapsed or refractory MM using the drug, Tazemetostat (EPZ-6438), which was developed by Epizyme. The study has been split into two parts. Part 1 involves a pharmacokinetics study of MM patients, without regard to BAP1 status, following a single drug dose. The second part of the study will recruit MM patients with BAP1-deficient MM tumors for continuous Tazemetostat treatment.
Conclusions
The steadily increasing number of reports of germline BAP1 mutations in high-risk cancer families has led to the discovery of a novel autosomal dominant, highly penetrant hereditary cancer syndrome that frequently predisposes to MM, UM, CM, atypical melanocytic tumors, and RCC, as well as other cancers such as basal cell carcinoma and meningioma. The tumor suppressor function of the BAP1 gene in MM has been definitively demonstrated genetically through in vivo experimental studies with Bap1-mutant mouse models. Although spontaneous MMs are rare in these mice, exposure to asbestos induced a highly significant increase in the incidence of aggressive MM in several different mouse models tested. Collectively, these findings provide genetic evidence that Bap1 is a bona fide tumor suppressor gene and offer key insights into the contribution of carcinogen exposure to enhanced cancer susceptibility. The continuing interest in elucidating mechanisms by which BAP1 inactivation contributes to cancer susceptibility and tumorigenesis has led to the discovery of a number of different BAP1 substrates and functions. Collectively, recent investigations suggest that BAP1 is a multifunctional protein that plays a role in cell cycle progression, DNA damage response/repair, and genomic instability in MM tumorigenesis. In turn, these novel findings have led to several proposed treatment options for this dreaded disease, such as the use of an EZH2 inhibitor in an ongoing clinical trial. Finally, the findings suggest that BAP1 mutation carriers who develop UM or atypical melanocytic tumors are at high risk of developing MM, CM or other cancers and, thus, should be closely monitored, with the goal of early intervention.
Acknowledgements
Funding: BAP1-related work performed by MC and JRT is supported by NCI grants CA175691 and CA06927, NIEHS grant P42 ES023720 (UPenn Superfund Research and Training Program Center), a grant from the Mesothelioma Applied Research Foundation—The Anderson Family Grant, an appropriation from the Commonwealth of Pennsylvania, and a gift from the Local #14 Mesothelioma Fund of the International Association of Heat and Frost Insulators & Allied Workers.
Footnote
Conflicts of Interest: JRT has served as a genetics consultant, and on one occasion, as an expert witness in a case involving the role of inherited mutations of BAP1 in mesothelioma. Both authors have a pending patent application on BAP1 genetic screening.
References
- Ai J, Stevenson JP. Current issues in malignant pleural mesothelioma evaluation and management. Oncologist 2014;19:975-84. [Crossref] [PubMed]
- Faig J, Howard S, Levine EA, et al. Changing pattern in malignant mesothelioma survival. Transl Oncol 2015;8:35-9. [Crossref] [PubMed]
- Bianchi AB, Mitsunaga SI, Cheng JQ, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A 1995;92:10854-8. [Crossref] [PubMed]
- Cheng JQ, Jhanwar SC, Klein WM, et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res 1994;54:5547-51. [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5. [Crossref] [PubMed]
- Jensen DE, Proctor M, Marquis ST, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16:1097-112. [Crossref] [PubMed]
- Machida YJ, Machida Y, Vashisht AA, et al. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem 2009;284:34179-88. [Crossref] [PubMed]
- Misaghi S, Ottosen S, Izrael-Tomasevic A, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol 2009;29:2181-92. [Crossref] [PubMed]
- Nishikawa H, Wu W, Koike A, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res 2009;69:111-9. [Crossref] [PubMed]
- Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 2010;465:243-7. [Crossref] [PubMed]
- Yu H, Mashtalir N, Daou S, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 2010;30:5071-85. [Crossref] [PubMed]
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410-3. [Crossref] [PubMed]
- Wiesner T, Obenauf AC, Murali R, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018-21. [Crossref] [PubMed]
- Abdel-Rahman MH, Pilarski R, Cebulla CM, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011;48:856-9. [Crossref] [PubMed]
- Ohar JA, Cheung M, Talarchek J, et al. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res 2016;76:206-15. [Crossref] [PubMed]
- de la Fouchardière A A, Cabaret O, Savin L, et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet 2015;88:273-7. [Crossref] [PubMed]
- Cheung M, Kadariya Y, Talarchek J, et al. Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene-environment interaction. Cancer Lett 2015;369:261-5. [Crossref] [PubMed]
- Wadt KA, Aoude LG, Johansson P, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet 2015;88:267-72. [Crossref] [PubMed]
- Pilarski R, Cebulla CM, Massengill JB, et al. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer 2014;53:177-82. [Crossref] [PubMed]
- Maerker DA, Zeschnigk M, Nelles J, et al. BAP1 germline mutation in two first grade family members with uveal melanoma. Br J Ophthalmol 2014;98:224-7. [Crossref] [PubMed]
- Ribeiro C, Campelos S, Moura CS, et al. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol 2013;24:2147-50. [Crossref] [PubMed]
- Popova T, Hebert L, Jacquemin V, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet 2013;92:974-80. [Crossref] [PubMed]
- Höiom V, Edsgärd D, Helgadottir H, et al. Hereditary uveal melanoma: a report of a germline mutation in BAP1. Genes Chromosomes Cancer 2013;52:378-84. [Crossref] [PubMed]
- Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res 2013;11:1061-71. [Crossref] [PubMed]
- Cheung M, Talarchek J, Schindeler K, et al. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet 2013;206:206-10. [Crossref] [PubMed]
- Aoude LG, Wadt K, Bojesen A, et al. A BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancers. PLoS One 2013;8:e72144. [Crossref] [PubMed]
- Aoude LG, Vajdic CM, Kricker A, et al. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res 2013;26:278-9. [Crossref] [PubMed]
- Wiesner T, Fried I, Ulz P, et al. Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol 2012;30:e337-40. [Crossref] [PubMed]
- Pilarski R, Rai K, Cebulla C, et al. BAP1 Tumor Predisposition Syndrome. In: GeneReviews(R), RA Pagon, MP Adam, HH Ardinger, et al. Editors. 2016: Seattle (WA).
- Evans DG, Ingham SL. Reduced life expectancy seen in hereditary diseases which predispose to early-onset tumors. Appl Clin Genet 2013;6:53-61. [Crossref] [PubMed]
- Lynch HT, Snyder C, Casey MJ. Hereditary ovarian and breast cancer: what have we learned? Ann Oncol 2013;24 Suppl 8:viii83-viii95.
- Hisada M, Garber JE, Fung CY, et al. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998;90:606-11. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407-16. [Crossref] [PubMed]
- Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868-74. [Crossref] [PubMed]
- Zauderer MG, Bott M, McMillan R, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 2013;8:1430-3. [Crossref] [PubMed]
- Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008;68:6953-62. [Crossref] [PubMed]
- Arzt L, Quehenberger F, Halbwedl I, et al. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res 2014;20:145-51. [Crossref] [PubMed]
- de Reyniès A, Jaurand MC, Renier A, et al. Molecular classification of malignant pleural mesothelioma: identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clin Cancer Res 2014;20:1323-34. [Crossref] [PubMed]
- De Rienzo A, Richards WG, Yeap BY, et al. Sequential binary gene ratio tests define a novel molecular diagnostic strategy for malignant pleural mesothelioma. Clin Cancer Res 2013;19:2493-502. [Crossref] [PubMed]
- Betti M, Aspesi A, Biasi A, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett 2016;378:120-30. [Crossref] [PubMed]
- Cheung M, Kadariya Y, Pei J, et al. An asbestos-exposed family with multiple cases of pleural malignant mesothelioma without inheritance of a predisposing BAP1 mutation. Cancer Genet 2015;208:502-7. [Crossref] [PubMed]
- Gupta MP, Lane AM, DeAngelis MM, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol 2015;133:881-7. [Crossref] [PubMed]
- Sahtoe DD, van Dijk WJ, Ekkebus R, et al. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat Commun 2016;7:10292. [Crossref] [PubMed]
- Yu H, Pak H, Hammond-Martel I, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A 2014;111:285-90. [Crossref] [PubMed]
- Ismail IH, Davidson R, Gagne JP, et al. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res 2014;74:4282-94. [Crossref] [PubMed]
- Dai F, Lee H, Zhang Y, et al. BAP1 inhibits the ER stress gene regulatory network and modulates metabolic stress response. Proc Natl Acad Sci U S A 2017;114:3192-7. [Crossref] [PubMed]
- Kadariya Y, Cheung M, Xu J, et al. Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline bap1 mutations. Cancer Res 2016;76:2836-44. [Crossref] [PubMed]
- Xu J, Kadariya Y, Cheung M, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res 2014;74:4388-97. [Crossref] [PubMed]
- Dey A, Seshasayee D, Noubade R, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012;337:1541-6. [Crossref] [PubMed]
- Napolitano A, Pellegrini L, Dey A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016;35:1996-2002. [Crossref] [PubMed]
- LaFave LM, Beguelin W, Koche R, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344-9. [Crossref] [PubMed]
- Shinozaki-Ushiku A, Ushiku T, Morita S, et al. Diagnostic utility of BAP1 and EZH2 expression in malignant mesothelioma. Histopathology 2017;70:722-33. [Crossref] [PubMed]
- Baughman JM, Rose CM, Kolumam G, et al. NeuCode proteomics reveals Bap1 regulation of metabolism. Cell Rep 2016;16:583-95. [Crossref] [PubMed]
- Gabitova L, Gorin A, Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res 2014;20:28-34. [Crossref] [PubMed]
- Riobo NA. Cholesterol and its derivatives in Sonic Hedgehog signaling and cancer. Curr Opin Pharmacol 2012;12:736-41. [Crossref] [PubMed]
- Parrotta R, Okonska A, Ronner M, et al. A novel BRCA1-Associated Protein-1 isoform affects response of mesothelioma cells to drugs impairing BRCA1-mediated DNA repair. J Thorac Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Krug LM, Kindler HL, Calvert H, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2015;16:447-56. [Crossref] [PubMed]
- Sacco JJ, Kenyani J, Butt Z, et al. Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors. Oncotarget 2015;6:13757-71. [Crossref] [PubMed]


