The clinical utility of circulating tumour cells in patients with small cell lung cancer
Small cell lung cancer (SCLC)
Lung cancer is one of the leading causes of cancer related mortality worldwide. SCLC accounts for 15% of all lung cancers and is characterised by rapid growth and early dissemination. Two thirds of patients present with metastatic disease (1) and despite initial exquisite chemo sensitivity, resistance emerges early and time to relapse is short, resulting in a dismal prognosis (2). Only 5% of patients diagnosed with SCLC are alive at 5 years.
Staging of SCLC was traditionally divided into limited (disease that can be encompassed in a single radiotherapy field) or extensive (metastatic) disease by The Veterans’ Administration Lung Group classification (3). This definition remains commonplace for routine treatment decision making and for clinical trial eligibility but is gradually being replaced by the tumour-node-metastasis (TMN) version 7 staging system, Union for International Cancer Control (UICC) classification, since subgroups with a distinct prognosis are identified within the limited stage group (4).
Surgery is rarely performed for SCLC, with evidence favouring the gold standard of combination chemotherapy to treat the expected micrometastatic disease and concurrent radiation treatment to optimise local control. In patients with extensive metastatic disease platinum/etoposide chemotherapy yields high response rates of 60–80% (5,6) and overall survival (OS) can be improved further with the adjunct of thoracic radiotherapy and prophylaxis cranial irradiation (1,7,8). The median OS in localised disease is 15.5 months and 20% of this group will have long term survival from treatment. However for the majority that relapse, further treatment options are limited. Topotecan is the only second line chemotherapy agent that is FDA approved for refractory or platinum resistant disease and the response rate is significantly lower in the second line setting, between 7–24% (2,9-13). In extensive stage SCLC the median OS is 10 months despite multimodality treatment (2).
Progress in understanding tumour biology and the development of precision medicine has lagged behind in SCLC compared to other tumour types (14). A barrier to progress undoubtedly has been the lack of good quality tumour samples available for basic and translational research. Diagnosis can be made on scant tumour samples or cytology alone and successful SCLC biopsies typically have extensive necrosis limiting their research utility (15).
Detection of circulating tumour cells (CTCs) in SCLC
CTCs have been identified and associated with poor prognosis in a range of cancer types. Numerous methods to enrich and enumerate these relatively rare cells from venous blood have been established allowing exploration of their clinical significance and relevance as a tumour biomarker (16-20).
Kularatne et al. utilised flow cytometry to detect CTCs in patients with SCLC in 2002 (21). Since this discovery both epitope dependent and epitope independent enrichment methods have detected an abundance of CTCs in SCLC patients, compared to other tumour types. Between 70–95% of patients with SCLC have detectable CTCs (22-30).
CTCs as a diagnostic biomarker in lung cancer
A key factor for improving outcomes in SCLC is early detection of disease amenable to curative intent. CT screening for individuals at high risk for developing lung cancer has not proven effective for detection of limited or early stage SCLC. In a trial of 54,454 patients who underwent low dose CT screening, 125 were diagnosed with SCLC. Of this group 86% had extensive metastatic disease at the time of diagnosis (31).
The exploration of CTCs as a tool for screening high risk individuals is in its infancy. In the context of non-small cell lung cancer (NSCLC), Ilie et al. performed screening in 245 patients at high risk of developing lung cancer, stratifying patients based on CTC detection. If CTCs were detected, patients entered a CT surveillance program. Of the 3% of patients with CTCs identified, 100% went on to develop pulmonary nodules over the following 1–4 years, that when resected proved to be early stage NSCLC. The remaining individuals in the CTC negative cohort did not develop lung cancer over the duration of follow up, indicating that CTCs could be a meaningful screening biomarker in NSCLC (32). However, at present there is no data for SCLC.
CTCs as a prognostic marker in SCLC
High CTC count is associated with poor OS and progression free survival (PFS) in a diverse range of cancers (16). Studies correlating CTC number with OS and PFS in metastatic breast, colorectal and prostate cancer, have resulted in the incorporation of CTC enumeration using the FDA approved CellSearch platform, into routine clinical practice (17,20,33).
In 2009, our laboratory used CellSearch to seek CTCs in the venous blood samples of 50 patients with SCLC: 86% had CTCs detectable (median number of 28), with a range of 0–44,896 CTCs identified. This was substantially higher than the numbers observed in other cancer types (34) demonstrating that SCLC generated an abundance of EpCAM expressing CTCs in the blood (22). In this exploratory analysis high numbers of CTCs (>300) were associated with reduced survival compared with fewer CTCs detected (<2) (22). Our group proceeded to explore the relationship between CTC number, prognosis and treatment. In a prospective study of 97 treatment naive SCLC patients, CTCs were enumerated at baseline and after one cycle of chemotherapy. Detected in 85% of patients, CTCs were found to be an independent prognostic factor for survival in univariate analysis. More than 50 CTCs at baseline was associated with a worse OS compared with those with less than 50 (median OS 5.4 vs. 11.5 months respectively; P<0.001) (23). Significant clinical factors for survival in the univariate analysis were stage, performance status, number of metastatic sites, treatment and lactate dehydrogenase. Adjusting for these factors in a multivariate analysis, CTC number was an independent prognostic factor for PFS (HR =2.01; 95% CI, 1.17–3.46; P=0.011) and OS (HR =2.45; 95% CI, 1.39–4.30; P=0.002) (23).
We also observed aggregates of CTCs, called circulating tumour microemboli (CTM) in 32% of patients who had detectable CTCs. These clusters of CTCs had a low proliferation index, assessed by immunohistochemistry Ki67 expression and absence of apoptotic morphology. Patients with even alone CTM, had a worse OS compared with CTM negative patients [median OS 4.3 months (95% CI, 0.87–7.7) vs. 10.4 months (95% CI, 9.0–11.7)] (23). CTCs have been observed to cluster with a range of different cell types and this may be a mechanism to form a defensive shield, avoiding direct interaction between natural killer cells and CTCs, preventing immune response (35). Platelets are suggested to be CTC protective, not only delivering barrier protection in the circulation but also providing key growth factors and cytokines (such as TGFβ) promoting a metastatic phenotype (36,37). Fibroblasts are seen in many primary tumour sites and it has been hypothesised that stromal cells in close proximity to CTCs may confer a survival advantage, aid transit in the bloodstream and influence the metastatic microenvironment (38). While the biology of CTM in SCLC is unclear their prognostic significance suggests that the cell to cell contact may afford protection and chemo resistance.
Several other studies support the prognostic utility of CTCs in SCLC. Hiltermann et al. (25) demonstrated in a study of 59 patients, that after stage, the presence of CTCs (≥2 per 7.5 mL of blood) was the strongest prognostic factor for OS (HR =3.1; 95% CI, 1.4–6.6; P≤0.001). Shi et al. used PCR quantification of CK-19 mRNA in venous blood to confirm the presence of CTCs. CTCs express CK-19 mRNA and their presence can be estimated by measuring CK-19 mRNA levels. The maximum observed value of CK-19 mRNA in controls with benign disease was 3.8. Patients with cancer and higher quantities of CK-19 mRNA were considered to be positive for CTCs. This was the case in 78.2% of patients who were deemed CTC positive at baseline. This group had a significantly shorter PFS and OS than the CTC negative group (P=0.014, 0.010 respectively) (29).
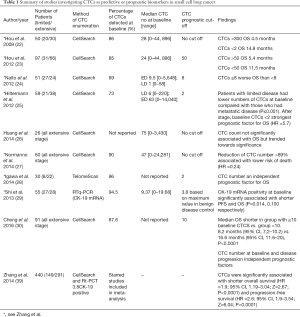
In considering the translation of these findings to routine clinical practice small sample size has been a substantial limitation, with the number of participants ranging from 26 to 97 in relevant studies (Table 1) (23,26). Zhang et al. presented a meta-analysis of seven studies conducted in 440 patients diagnosed with SCLC which supported the prognostic significance of CTCs. They concluded that the presence of CTCs (≥2) was significantly associated with reduced OS (HR =1.9; 95% CI, 1.19–3.04; Z=2.67; P<0.0001) and reduced PFS (HR =2.6; 95% CI, 1.9–3.54; Z=6.04; P<0.0001) (39).
Full table
Methods used to detect CTCs vary across studies, as do the methods employed to identify significant CTC thresholds (40,41). Naito et al., using the maximal hazards ratio, identified a threshold of ≥8 CTCs, detected by CellSearch, as significantly predictive of OS (HR =3.5; 95% CI, 1.45–8.6; P=0.0014), with 78% of patients that had <8 CTCs at baseline surviving I year compared with only 31.6% of those with ≥8 CTCs detected (24). Longitudinal samples revealed that a CTC count ≥8 after treatment and at relapse indicated a worse prognosis compared with those who had <8 CTCs at these time points (P=0.0096 for post treatment and P<0.0001 for relapse) (24).
Cheng et al. applied time dependent receiver operating characteristics (ROC) analysis to identify significant CTC thresholds in their study of 91 treatment naive SCLC patients’ randomised patients to two different chemotherapy regimens. They concluded that 10 CTCs detected by CellSearch appeared to be the optimal cut off for predicting PFS and OS. Multivariate analysis demonstrated that baseline CTC count was prognostic for OS (HR =0.304; P<0.0001) and that <10 CTCs at baseline and disease progression predicted a significantly improved median OS (30).
Our group identified a threshold of 50 CTCs as the most significant discrimination in survival estimations when testing a series of baseline CTC values using the Kaplan-Meier method and applying Bonferroni correction for multiple testing (42). Analysis of ROC curves confirmed 50 CTCs to be the optimal cut off (23).
An arbitrary threshold of 2 CTCs was identified as significant by Igawa et al. (28) who used a novel OBP-401 assay to identify CTCs in SCLC patients. This telomerase-specific replication selection adenovirus identified CTCs on the principal that immortal cancers cells are known to maintain telomere length, resulting in cell proliferation and evading replicative senescence. Telomerase activity is observed in cancer cell lines, maintaining telomerase length, but there is minimal activity in normal healthy cells (43). The assay identified telomerase activity utilising immunocytochemical analysis to identify individual cells. In multivariate analysis CTC number was an independent prognostic factor for survival (HR =3.91; P=0.026). Patients with <2 CTC at baseline had longer median survival than those with ≥2 (14.7, 95% CI, 11.5–18.2 vs. 3.9, 95% CI, 3.3–4.6; P=0.007) and patients with <2 CTCs at two cycles of chemotherapy tended to have a longer median PFS [8.3 (95% CI, 5.3–11.3) vs. 3.8 (95% CI, 2.5–5.0); P=0.07]. Regardless of enumeration technique and statistical methods applied baseline pre-treatment CTC number consistently correlates with OS.
CTCs as a predictive marker biomarker in SCLC
The use of baseline CTCs as a predictive biomarker for treatment response (per RECIST criteria) was incorporated into a study design by Wang et al. (44). In this study 96 patients who received chemo-radiation for limited or extensive SCLC had baseline CTC enumeration by CellSearch. No association could be found between any baseline CTC thresholds and treatment response. This may suggest that although baseline CTC count is a proven prognostic biomarker for OS and PFS, baseline CTC count does not predict for response.
However a change in CTC count during treatment at time of progression may provide prognostic significance. Normanno et al. compared CTC count at baseline and after one cycle of treatment in 40 patients with metastatic SCLC and observed that a reduction in count of >89% was significantly associated with a lower risk of death (HR =0.24; 95% CI, 0.09–0.61). The authors concluded that early reduction in CTC count improved estimations of prognosis and that change in CTC number was significantly more useful than baseline count alone (27). Hiltermann and colleagues’ study, which included 59 patients with SCLC and enumerated blood samples at baseline (73% had CTC’s detected), post cycle 1 (21.6% had CTCs detected) and cycle 4 (20.6% CTCs detected), compared CTC trend to radiological response on CT imaging. A multivariate Cox regression analysis revealed that a decrease in CTC count post chemotherapy was a stronger predictor for OS (P=0.004) than disease stage or CT response (25).
These studies are consistent with our own analysis in which CTC number following cycle 1 of chemotherapy provided additional prognostic information. Low CTC number (<50) after one cycle of chemotherapy was associated with a significantly longer PFS (9.6 months; 95% CI, 7.8–11.5 months) and OS (10.4 months; 95% CI, 8.8–11.9 months) compared with those who had ≥50 CTCs post treatment (PFS 4.1 months (95% CI, 0–9.2); OS 4.1 months (95% CI, 0–8.5) (23). A fall in CTC count was associated with tumour shrinkage and better outcomes highlighting the potential of CTCs to be used as a pharmacodynamic biomarker for response. Taken together these results argue for CTC number to be a stratification factor in clinical trials for patients with SCLC. CTC number at baseline and following a cycle of treatment also could aid in refining prognostic information crucial for the patient to make informed decisions when discussing goals of care in the palliative setting (45,46).
Molecular characterisation of CTCs in patients, beyond CTC enumeration, can be combined with other biomarkers to provide more accurate clinical information. Pore et al hypothesised that the presence of cancer stem cell markers and mesenchymal markers in tumour biopsy samples could have a relationship to the number of CTCs and OS (47). Tumour samples were taken from 38 patients and immunohistochemical staining for mesenchymal markers and cancer stem cell markers performed. Again, CTC number was the strongest predictor of survival, over disease stage or marker expression [≥2 CTCs associated with a significantly worse prognosis than those with <2 (HR =3.43; 95% CI, 1.46–8.03; P=0.005)]. The addition of immunohistochemical markers to CTC number did not significantly change the HR. However, the authors did observe that expression of a ‘mesenchymal like’ profile of c-METHE-cadL was associated with a significantly better prognosis (OS HR 0.30; 95% CI, 0.13–0.72) and in this small group there was a trend towards lower baseline CTCs (P=0.09).
Applying CTC enumeration to clinical trials in patients with SCLC
Several clinical trials conducted in patients with SCLC have now incorporated CTC enumeration as an exploratory biomarker for prediction of treatment response or resistance. Belani et al. (48) enumerated baseline CTCs in a randomised phase II trial that combined vismodegib (a hedgehog inhibitor) or cixutumumab (an insulin like growth factor 1 receptor inhibitor) with standard chemotherapy for metastatic SCLC. Amongst the 168 patients recruited, 120 had CTCs enumerated at baseline and 32.5% of these patients had a baseline CTC count >100. High CTCs (>100) were prognostic; associated with worse OS [median OS 7.2 months (95% CI, 6.4–8.5) vs. 10.5 months (95% CI, 9.4–13.2)] estimated OS HR 1.76 (P=0.005; 95% CI, 1.18–2.63). There was no significant difference between the treatment groups, nor any association between baseline CTC count and response.
A promising utility of CTC enumeration in early phase trials is for serial monitoring as a surrogate for response. Pietanza et al. (49) incorporated CTC enumeration into a phase 1 trial of sonidegib (a hedgehog pathway inhibitor) in combination with cisplatin and etoposide in a cohort of 15 patients with extensive SCLC. CTCs were enumerated at baseline, at each cycle of chemotherapy, every 3 months on maintenance therapy and at disease progression. Baseline CTCs in 14 patients ranged from 0 to >200. Again univariate analysis confirmed that baseline CTC count was associated with worse OS, with a median OS for patients with CTCs >200 of 6.2 months compared with 25.7 months for the patients with <200 CTC. Detectable CTCs at cycle 2 day 1 were associated with worse survival, with a median OS of 5.5 months for detectable CTCs. From the treatment cohort, 79% of patients had a partial response and in this group 10 of the 11 patients had zero CTCs at time of the first follow up. This is in contrast to the patients who had stable disease, all of which had CTCs detectable on follow up. Of note a rise in CTC count from nadir to time of progression was observed in 5 of the 13 patients. CTC enumeration could therefore have a valuable role in identifying non-responders early, potentially sparing unnecessary toxicity and allowing opportunity for treatment change prior to clinical deterioration.
Messaritakis et al. molecularly characterised CTCs from SCLC patients into three distinct subpopulations; TTF1+ & CD45−, CD56+ & CD45−, TTF1+ & CD56−. They then explored the changes in subpopulation proportions longitudinally during treatment with the experimental agent pazopanib. Of the 58 patients with evaluable CTCs 8% had one sub population identified, 36.2% two subpopulations identified and 27.6% three subpopulations at baseline. On serial assessment there was no significant difference in the detection rate of the different CTC subpopulations after one cycle of pazopanib or on PD. However, patients with PD had a significantly higher number of CTCs at baseline compared to patients with PR or SD [PD: median 17 CTCs/7.5 mL (range, 0–11.143) vs. PR: median 0 CTCs/7.5 mL (range, 0–388) vs. SD: median 2 CTCs/7.5 mL (range, 0–27) respectively; P=0.006], suggesting that there may be a role for CTCs in predicting drug resistance to pazopanib (50).
CTCs in limited stage SCLC
The clinical utility of CTCs in limited stage SCLC is yet to be fully explored. There are many unanswered questions such as whether CTCs can assist with identification of the select few who would benefit from curative surgery (51). Conversely, can CTC enumeration identify a group of patients with limited disease who would benefit most with respect to long term survival and cure, from aggressive concurrent chemo radiation with curative intent or conversely can some individuals be spared this demanding treatment if they will inevitably relapse in a short time frame? Recently presented data from the concurrent once daily (od) vs. twice daily (bd) RadioTherapy (CONVERT) study, that compared once daily radiotherapy to twice daily radiotherapy in limited stage SCLC, provides CTC data for a subgroup of 75 patients at our institute. CTC number was highly prognostic for survival and the most significant threshold to discriminate good from poor outcomes was >15 CTCs/7.5mL of blood. All patients with >15 CTC at baseline progressed and died within 2 years of follow up (52). The median OS of all patients on the study was 30 months (95% CI, 24–34 months) in the twice daily treatment group and 25 months (95% CI, 21–31 months) in the once daily treatment group, which was not a statistically significant difference (53). Studies such as this will aid future patient stratification in clinical trials (52,54).
Molecular characterisation of CTCs in SCLC
CTCs are tumorigenic and our group has successfully demonstrated that CTCs isolated from a proportion of patients with SCLC can generate patient CTC derived explants (CDX) when injected into immunodeficient mice (55). Molecular testing of these tumours has shown a high degree of similarity in CNA (copy number alteration) patterns to the donor CTCs and CDX models recapitulate the responses to chemotherapy observed in the donor patients. Paired CDX models from patients at baseline and again at relapse have been derived to enable preclinical drug testing and analysis of molecular characteristics of response and resistance (55). The ultimate hope of CTC analysis from patients with SCLC would be identification biological mechanisms that can be exploited for therapeutic control and relevant biomarkers for precision medicine approaches.
Studies to explore CTC heterogeneity towards better understanding of CTC biology and treatment resistance are ongoing (56). To this end single CTCs can be isolated for genomic profiling. Our group has identified a CNA classifier predictive of chemo response that applied to 200 CTCs from 31 patients, accurately predicts chemo refractory or chemo sensitive disease in 83.3% of patients (57). However the same CNA classifier does not predict for acquired resistance in longitudinal samples taken from individuals suggesting an alternative resistance mechanism (57). Appreciating genomic, transcriptome and epigenetic variation plus proteomic analysis at a single cell level at diagnosis, during emerging treatment resistance and the development of new sites of metastatic disease is required to further understanding of the evolution of cancer and treatment resistance mechanisms.
Perspectives
CTC enumeration and molecular characterisation in SCLC is beginning to improve knowledge of SCLC biology in this recalcitrant cancer. There is potential for CTC enumeration to aid patient decision making in the clinic. CTC enumeration should be incorporated into clinical trials, as a stratification factor given its independent prognostic value to avoid bias in survival analyses in small early phase trials. Monitoring of CTCs may aid identification of early relapse and treatment resistance providing opportunity for therapeutic change in the narrow window of opportunity prior to symptomatic decline. CTC enumeration could also be used as a pharmacodynamic biomarker in drug development and guide optimal therapeutic dosing revolutionising the current, relatively crude method, of maximum tolerated dose.
Conceivably the most impactful developments will result from the molecular characterisation of CTCs. Genomic profiling of CTCs longitudinally has potential to inform on mechanisms of chemo resistance. Moreover, molecular characterisation of CTCs could be used for treatment selection as relevant predictive biomarkers as effective precision medicines evolve. As a case in point an assay to identify patients for DLL3 targeted Rova-T is currently in clinical development (58). CTCs now provide a ‘liquid biopsy’ that allows repeated scrutiny of tumour characteristics and offers unprecedented potential to tailor a truly dynamic personalised medicine approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Johnson BE, Grayson J, Makuch RW, et al. Ten-year survival of patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. J Clin Oncol 1990;8:396-401. [Crossref] [PubMed]
- Foster NR, Renfro LA, Schild SE, et al. Multitrial Evaluation of Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Extensive-Stage Small-Cell Lung Cancer. J Thorac Oncol 10:1099-106. [Crossref] [PubMed]
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973;4:31-42. [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471-7. [Crossref] [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. First-line therapy with VP-16 and cisplatin for small-cell lung cancer. Semin Oncol 1986;13:17-23. [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [Crossref] [PubMed]
- Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007;2:348-54. [Crossref] [PubMed]
- Nosaki K, Seto T. The Role of Radiotherapy in the Treatment of Small-Cell Lung Cancer. Curr Treat Options Oncol 2015;16:56. [Crossref] [PubMed]
- Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162-9. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. [Crossref] [PubMed]
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623-31. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [Crossref] [PubMed]
- Dago AE, Stepansky A, Carlsson A, et al. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS One 2014;9:e101777. [Crossref] [PubMed]
- Harouaka R, Kang Z, Zheng SY, et al. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther 2014;141:209-21. [Crossref] [PubMed]
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218-24. [Crossref] [PubMed]
- Kularatne BY, Lorigan P, Browne S, et al. Monitoring tumour cells in the peripheral blood of small cell lung cancer patients. Cytometry 2002;50:160-7. [Crossref] [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-16. [Crossref] [PubMed]
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [Crossref] [PubMed]
- Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref] [PubMed]
- Huang CH, Wick JA, Sittampalam GS, et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front Oncol 2014;4:271. [Crossref] [PubMed]
- Normanno N, Rossi A, Morabito A, et al. Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer. Lung Cancer 2014;85:314-9. [Crossref] [PubMed]
- Igawa S, Gohda K, Fukui T, et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol Lett 2014;7:1469-73. [PubMed]
- Shi WL, Li J, Du YJ, et al. CK-19 mRNA-positive cells in peripheral blood predict treatment efficacy and survival in small-cell lung cancer patients. Med Oncol 2013;30:755. [Crossref] [PubMed]
- Cheng Y, Liu XQ, Fan Y, et al. Circulating tumor cell counts/change for outcome prediction in patients with extensive-stage small-cell lung cancer. Future Oncol 2016;12:789-99. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Ilie M, Hofman V, Long-Mira E, et al. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 2014;9:e111597. [Crossref] [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Lou XL, Sun J, Gong SQ, et al. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res 2015;27:450-60. [PubMed]
- Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-4. [Crossref] [PubMed]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. [Crossref] [PubMed]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 2009;9:285-93. [Crossref] [PubMed]
- Zhang J, Wang HT, Li BG. Prognostic significance of circulating tumor cells in small--cell lung cancer patients: a meta-analysis. Asian Pac J Cancer Prev 2014;15:8429-33. [Crossref] [PubMed]
- Leeflang MM, Deeks JJ, Takwoingi Y, et al. Cochrane diagnostic test accuracy reviews. Syst Rev 2013;2:82. [Crossref] [PubMed]
- Riley RD, Ahmed I, Ensor J, et al. Meta-analysis of test accuracy studies: an exploratory method for investigating the impact of missing thresholds. Syst Rev 2015;4:12. [Crossref] [PubMed]
- Boulesteix AL. Maximally Selected Chi-Square Statistics and Binary Splits of Nominal Variables. Biometrical Journal 2006;48:838-48. [Crossref] [PubMed]
- Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011-5. [Crossref] [PubMed]
- Wang X, Ma K, Wang Y, et al. Evaluation of Circulating Tumor Cells in Predicting Therapeutic Response in Small Cell Lung Cancer Patients. Arch Med Res 2016;47:454-9. [Crossref] [PubMed]
- Temel JS, Greer JA, Admane S, et al. Longitudinal Perceptions of Prognosis and Goals of Therapy in Patients With Metastatic Non–Small-Cell Lung Cancer: Results of a Randomized Study of Early Palliative Care. J Clin Oncol 2011;29:2319-26. [Crossref] [PubMed]
- Innes S, Payne S. Advanced cancer patients’ prognostic information preferences: a review. Palliat Med 2009;23:29-39. [Crossref] [PubMed]
- Pore M, Meijer C, de Bock GH, et al. Cancer Stem Cells, Epithelial to Mesenchymal Markers, and Circulating Tumor Cells in Small Cell Lung Cancer. Clin Lung Cancer 2016;17:535-42. [Crossref] [PubMed]
- Belani CP, Dahlberg SE, Rudin CM, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 2016;122:2371-8. [Crossref] [PubMed]
- Pietanza MC, Litvak AM, Varghese AM, et al. A phase I trial of the Hedgehog inhibitor, sonidegib (LDE225), in combination with etoposide and cisplatin for the initial treatment of extensive stage small cell lung cancer. Lung Cancer 2016;99:23-30. [Crossref] [PubMed]
- Messaritakis I, Politaki E, Plataki M, et al. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer 2017;104:16-23. [Crossref] [PubMed]
- Hamilton G, Rath B, Ulsperger E. A review of the role of surgery for small cell lung cancer and the potential prognostic value of enumeration of circulating tumor cells. Eur J Surg Oncol 2016;42:1296-302. [Crossref] [PubMed]
- Fernandez-Gutierrez F, Foy V, Burns K, et al. Prognostic value of Circulating Tumour Cells in Limited-Disease Small Cell Lung Cancer Patients treated on the CONVERT trial IASLC. J Thorac Oncol 2017;12:S263. [Crossref]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Faivre-Finn C, Falk S, Ashcroft L, et al. Protocol for the CONVERT trial-Concurrent ONce-daily VErsus twice-daily RadioTherapy: an international 2-arm randomised controlled trial of concurrent chemoradiotherapy comparing twice-daily and once-daily radiotherapy schedules in patients with limited stage small cell lung cancer (LS-SCLC) and good performance status. BMJ Open 2016;6:e009849. [Crossref] [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [Crossref] [PubMed]
- Williamson SC, Metcalf RL, Trapani F, et al. Vasculogenic mimicry in small cell lung cancer. Nat Commun 2016;7:13322. [Crossref] [PubMed]
- Carter L, Rothwell DG, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med 2017;23:114-9. [Crossref] [PubMed]
- Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42-51. [Crossref] [PubMed]


