Update on large cell neuroendocrine carcinoma
Introduction
Lung tumors with neuroendocrine morphology by light microscopy consist of a spectrum of tumor types with different biology and clinical features. The morphologic types include low-grade typical carcinoid, intermediate-grade atypical carcinoid and high-grade neuroendocrine carcinomas that are further classified into large cell neuroendocrine carcinoma (LCNEC), and small cell lung carcinoma (SCLC). Growing evidence suggests that carcinoid tumors are biologically distinct from high-grade neuroendocrine carcinomas given the significant differences in clinical behavior and molecular alterations as well as possible precursor lesions and association with other histologic types between the two groups (1-5).
Of the high-grade neuroendocrine carcinomas, SCLC harbors a distinct biology and clinical course. SCLC is found almost exclusively in smokers and has a rapid doubling time and a more aggressive clinical course than non-small cell lung cancer (NSCLC), with the majority presenting at stage IV. Interestingly, Govindan et al., using the surveillance, epidemiology and end results database, have reported a decrease in the incidence of SCLC (as a percentage of the number of patients diagnosed with all types of lung cancer) from 17.26% in 1986 to 12.95% in 2002 (6). While a decrease in the incidence of smoking and the change to low-tar filter cigarettes likely contribute to the decreasing incidences of SCLC, they may be attributed in part to how pathologists distinguish between SCLC and NSCLC, in particular LCNEC (7).
Pathological diagnosis of LCNEC is challenging in some cases, and it is essentially diagnosed on resected specimens. The incidence of LCNEC is very low and has been reported to range from 2.4% to 3.1% in resected lung cancers (8,9). Patients with LCNEC are predominantly male (8,10), older, and heavy smokers (8,9). All-stage 5-year overall survival for LCNEC ranges from 13% to 57% (11), and the prognosis of LCNEC is comparable to that of SCLC (10,12). Even patients with pathologic stage IA LCNEC treated by complete resection have worse outcomes than those with non-neuroendocrine NSCLC (13). However, optimal therapy for advanced LCNEC has not been defined yet. Several studies have evaluated response to chemotherapy and/or radiation in patients with advanced LCNEC, the diagnosis of which was made on biopsy samples, leading to conflicting results as to whether LCNEC is responsive to platinum/etoposide that is typically effective for SCLC (14-18). It may be explained by biological heterogeneity of LCNEC that has been elucidated by the recent molecular studies revealing a few subsets in LCNEC (19,20). In this review, we will discuss the clinical, pathological, and molecular aspects of LCNEC to elucidate its heterogeneous nature.
Pathological features
Histologically, LCNEC is defined as a non-small cell carcinoma with neuroendocrine morphology including organoid or trabecular patterns, rosette-like structures or peripheral palisading, as well as neuroendocrine differentiation confirmed by immunohistochemistry and/or electron microscopy (Figure 1) (21). Tumor cells are typically greater than 3 times the diameter of resting lymphocytes and exhibit moderate, often eosinophilic, cytoplasm and prominent nucleoli (22,23). Mitotic activity is usually brisk (21), and large areas of necrosis are typically seen. One unequivocally positive neuroendocrine marker (synaptophysin, chromogranin A or CD56) is sufficient to confirm the diagnosis, but CD56 expression alone must be interpreted with caution (24-26). The differentiation of LCNEC from its mimickers, including small cell carcinoma, atypical carcinoid, and NSCLC, can be challenging, in particular in biopsy/cytology specimens. Thus the diagnosis of LCNEC can only be made in resection specimens, while it could be suggested in the small samples.

SCLC vs. LCNEC
The classic neuroendocrine morphology may be seen in SCLC, but is uncommon, in particular, on the small biopsies typically obtained from these patients. The latter often exhibit a sheet-like growth without the neuroendocrine morphology. Tumor cells of SCLC are round, oval, or spindle-shaped; they usually are less than the size of three small resting lymphocytes in biopsy specimens, but there is no absolute cutoff for tumor cell size and they often appear larger in resection specimens (27-29). More importantly, SCLC is characterized by scant cytoplasm, a high N/C ratio, finely granular chromatin, and absent or inconspicuous nucleoli (Figure 2). The mitotic rate is high and averages over 60 mitoses per 2 mm2 (27,28). Necrosis is common and often extensive. Basophilic staining of vascular walls due to encrustation by DNA from necrotic tumor cells (the Azzopardi phenomenon) is frequent in areas of necrosis. If tumor cells with conspicuous nucleoli or pleomorphic giant tumor cells are identified, these are interpreted as large cell carcinoma elements, and when these constitute 10% or more of the tumor volume, the tumor is diagnosed as combined small cell and LCNEC (28,29).
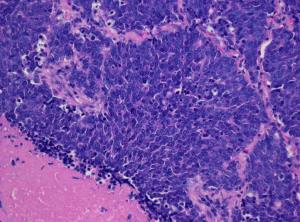
Although typical LCNEC is morphologically distinct from SCLC, the differentiation between LCNEC and SCLC can be difficult in some cases. In the study by Travis et al. in which surgically resected neuroendocrine tumors of the lung were reviewed independently by five pulmonary pathologists, there was unanimous diagnostic agreement only on 70% of SCLCs and 40% of LCNECs, and most of disagreements fell between LCNEC and SCLC (30). The difficulty in differentiating between the two entities may be attributed in part to considerable overlap in cytomorphology (29). For instance, Marchevsky et al. reported that there was considerable overlap in nuclear size between SCLC and LCNEC (22).
Upon reviewing the histologic features of high-grade neuroendocrine carcinomas, we also found a group of tumors temporarily categorized as borderline high-grade neuroendocrine carcinomas that fell between LCNEC and SCLC (31). They are characterized by tumor cells with a relatively small amount of cytoplasm, a high N/C ratio, finely granular chromatin, and small or inconspicuous nucleoli (features of SCLC) and a polygonal shape, large nuclei and organoid, trabecular, palisading patterns, and/or a rosette-arrangement (features of LCNEC) (Figure 3). A morphometric study confirmed that tumor nuclear diameter/lymphocyte size (T/L) ratios of the borderline category (2.91±0.76) fell between those of SCLC (2.62±0.90) and LCNEC (3.22±0.86) (31,32). Interestingly, borderline high-grade neuroendocrine carcinomas have a CD56+, mASH1+, chromogranin A-, synaptophysin- immunophenotype, and TTF-1 tends to be negative (31).

Asamura et al. also reported 14 borderline high-grade neuroendocrine carcinomas, which comprised 5.5% of the 254 high-grade neuroendocrine carcinomas in their multi-institutional study (10). We hypothesize that the presence of borderline high-grade neuroendocrine carcinomas contributes to the discordant classification of high-grade neuroendocrine carcinomas among pathologists (31-33). Some pathologists may classify borderline high-grade neuroendocrine carcinomas as SCLC based on the high N/C ratio and the relatively scant cytoplasm. For example, Figure 10 in the article by Nicholson et al. represents borderline high-grade neuroendocrine carcinoma according to our criteria, but they diagnosed it as SCLC (29). Conversely, some pathologists may classify the same case as LCNEC based on the polygonal shape of tumor cells, larger nuclei, and conspicuous nucleoli.
Atypical carcinoid tumor vs. LCNEC
While the majority of carcinoid tumors harbor low mitotic rates that fall in the range defined by the WHO classification (≤10 per 2 mm2) (34), we rarely encounter tumors with classic carcinoid morphology and mitotic rates exceeding 10 mitoses per 2 mm2 (35-38).The current WHO scheme recommends that the latter be classified as LCNEC (21). Quinn et al. reported 12 lung neuroendocrine tumors with morphologic features of carcinoid and mitotic counts of >10 per 2 mm2. All but one patient developed metastasis, and seven died of the tumor. Thus, they concluded that this subset of tumor appears aggressive and would be classified as LCNEC according to the current WHO classification scheme. However, clinical and pathologic features of these tumors appear to be more in common with carcinoid tumors than LCNEC.
LCNEC vs. NSCLC
Adenocarcinomas with solid pattern may show a nesting pattern and large areas of necrosis, mimicking LCNEC. Central luminal spaces surrounded by tumor cells observed in adenocarcinomas with cribriform pattern may be confused with rosette-like structures of LCNEC. However, they can be differentiated from LCNCE by the absence of neuroendocrine marker expression. Basaloid squamous cell carcinoma shows nesting and/or trabecular growth patterns with peripheral palisading and large areas of necrosis, mimicking LCNEC. Some basaloid squamous cell carcinomas have very small tumor cells that can resemble those of SCLC. However, the diffuse, strong p40 and CK5/6 positivity that characterize basaloid squamous cell carcinoma is absent in LCNEC and SCLC. Conversely, unequivocal neuroendocrine differentiation by immunohistochemistry and/or EM, characteristic of most LCNEC and SCLC, is not seen in basaloid squamous cell carcinoma. Large cell carcinomas with neuroendocrine morphology and negative neuroendocrine markers are referred to large cell carcinoma with neuroendocrine morphology (LCCNM), while large cell carcinomas which do not show neuroendocrine morphology by light microscopy but do demonstrate neuroendocrine differentiation on immunohistochemistry and/or EM are referred to large cell carcinoma with neuroendocrine differentiation (LCCND). LCCNM and LCCND are not included in the LCNEC category. Although their biology has not been fully examined due to the small numbers of such entities, some studies have reported that they are as aggressive as LCNEC (8,12). The presence of morphologic overlap between LCNEC and the well-defined entities is likely reflection of its biologic heterogeneity reported by the recent molecular studies (19,20). For instance, using next-generation sequencing (NGS), Rekhtman and colleagues have identified two major and one minor subsets in LCNEC, namely SCLC-like, NSCLC-like and carcinoid-like subsets. The genomically defined subgroups were enriched in tumors with SCLC-like, NSCLC-like and carcinoid-like morphologic features, respectively, and as a group, tumors with SCLC-like molecular features were more proliferative, although there is a substantial histologic overlap between the SCLC-like and NSCLC-like subsets (19).
Molecular features
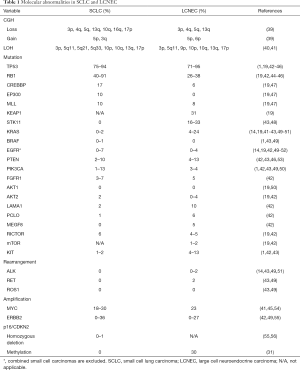
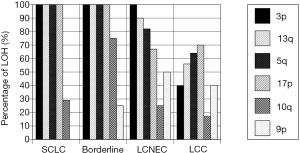
There are multiple studies that have shown similarities and differences in molecular alterations among high-grade neuroendocrine carcinoma (Table 1). Genome-wide high-resolution search for loss of heterozygosity (LOH) in high-grade neuroendocrine carcinomas has been conducted using SCLC and NSCLC cell lines (57,58). These studies found that LOH of some loci was mutual in SCLC and NSCLC subtypes, whereas LOH of some other loci was subtype-specific. In our study, all of SCLC and borderline high-grade neuroendocrine carcinomas showed LOH at 3p, 13q, and 5q (Figure 4), while LOH at 5q and 17p was more prevalent in SCLC and borderline cases than in LCNEC, and LOH at 9p was more frequent in LCNEC (31). As for copy number alterations, losses of 3p, 4q, 5q, and/or 13q and a gain of 5p were seen in both SCLC and LCNEC, while a gain of 3q and losses of 10q, 16q, and/or 17p were observed frequently in SCLC but not in LCNEC, and a gain of 6p occurred more frequently in LCNEC (39).
Full table
Jones et al. investigated gene expression profiles using cDNA microarrays with over 40,000 elements and were able to divide LCNECs and SCLCs into two groups according to prognosis. However, they did not correspond to LCNECs and SCLCs as defined histologically (59).
At an individual gene level, LCNEC shares TP53 and RB1 mutations with SCLC (60). However, the frequency of RB1 mutations is lower in the LCNEC than in SCLC (42). Conversely, SKT11, KRAS, KEAP1, LAMA1, PCLO, and MEGF8 mutations are more prevalent in LCNEC than in SCLC (19,42). EGFR mutations are restricted to a very minor component of both SCLCs and LCNECs (14,42,49-52), but they may be seen more frequently in combined histology. For instance, one study has reported EGFR mutations in 3 of 15 (20%) combined SCLCs with adenocarcinoma (52). ALK, ROS1, BRAF, RET, or ERBB2 alterations are very rarely detected in both SCLC and LCNEC (1,14,42,43,49,51,55), while c-MYC amplification were observed in less than 1/3 of both SCLC and LCNEC (41,45,54). Inactivation of p16/CDKN2, via homozygous deletions, mutations or promoter hypermethylation, has been reported in LCNEC and borderline high-grade neuroendocrine carcinomas, but is vanishingly rare in SCLC (31,56,61), in which RB mutations are common and do not require p16/CDKN2 inactivation for CDK4 activation (61).
As for expression of biomarkers, Bari et al. performed high-throughput gene expression profiling in eight LCNEC and eight SCLC samples and revealed relative up-regulation of CDX2 and villin in LCNEC and that of BAI3 in SCLC (62).
These similarities and differences in molecular profiles between LCNEC and SCLC can be explained by the heterogeneous biology of LCNEC that has recently been elucidated by two studies (19,20). Fernandez-Cuesta and colleagues analyzed 60 LCNEC cases and confirmed the presence of two well-defined groups of LCNEC: a SCLC-like group, carrying MYCL1 amplifications and concurrent RB1 and TP53 mutations; and an adenocarcinoma/squamous cell carcinoma-like group, harboring p16/CDKN2A deletions, TTF-1 amplifications, and frequent mutations in KEAP1 and STK11 (20). Similarly, in the aforementioned study, Rekhtman and colleagues analyzed 45 LCNEC cases with NGS of 241 cancer genes and found two major molecular subsets along with a minor subset. The major subsets consisted of SCLC-like LCNEC (n=18), characterized by TP53 and RB1 co-mutation/loss and other SCLC-type alterations, including MYC amplification, and NSCLC-like LCNEC (n=25), characterized by lack of concurrent TP53 and RB1 alterations and nearly universal occurrence of NSCLC-type mutations (SKT11, KRAS, and/or KEAP1). In addition, they identified two carcinoid-like tumors, characterized by MEN1 mutations and low mutation burden (19).
Therapy
Dresler et al. reported no survival benefits from postoperative chemotherapy, radiation therapy, or both in patients with resected LCNEC (63). Iyoda et al., however, have shown that adjuvant chemotherapy improved survival in stage I LCNEC but not in stage II or III disease in a retrospective study (64). The study by Rossi et al. reported the role of a SCLC-based chemotherapy in an adjuvant setting for stage I–III diseases (11). Iyoda et al., in their recent prospective study, concluded that adjuvant chemotherapy, consisting of platinum/etoposide, after surgery appeared preventing recurrence in patients with completely resected LCNECs (65). While surgery with a curative intent and completeness of resection may be advocated for the treatment of patients with LCNEC, they will likely benefit from adjuvant chemotherapy with a SCLC regimen (66).
In the advanced stage setting, while response rates of approximately 50% and up to 80% have been demonstrated in prospective studies utilizing platinum/etoposide in patients with extensive stage SCLC, chemosensitivity of LCNEC to this regimen is somewhat controversial (14-18,67). It may be attributed in part to the heterogeneous morphology and biology of LCNEC. In addition, the difficulty in diagnosing LCNEC on biopsy specimens may lead to the conflicting results. Among several retrospective studies, Sun et al. reviewed 45 patients with advanced LCNEC and concluded that advanced LCNEC can be appropriately treated in the similar manner to SCLC rather than NSCLC (18). In this cohort, eleven patients received first-line platinum/etoposide, and the remaining 34 patients received various types of NSCLC regimens. The response rate to the SCLC regimen was 73%, which was higher than that (50%) to NSCLC regimen. The median overall survival was 16.5 and 9.2 months in the SCLC and NSCLC regimen groups, respectively. Of note, pathological data were extracted from medical records; however, it is not clear how the pathological diagnosis of LCNEC were rendered. Similarly, Shimada et al. found that overall response rate to the initial chemotherapy or chemoradiotherapy and the survival outcomes of high-grade neuroendocrine carcinoma—probable LCNE were comparable to those of SCLC. In their study, 25 patients, who had been diagnosed to have high-grade neuroendocrine carcinoma-probable LCNEC by review of biopsy specimens and had undergone chemotherapy or chemoradiotherapy as the initial therapy, were compared with 180 patients with SCLC (17). Yamazaki et al. have also reported that the response rate of LCNEC to platinum-based chemotherapies was comparable to that of SCLC in their review of 20 patients with stage IIIA–IV LCNEC. In this study, pathological diagnosis of LCNEC was confirmed with operation or autopsy. The patients received a combination of platinum/etoposide (n=9), platinum, vindesine and mitomycin (n=6), platinum and vindesine (n=4), or platinum alone (n=1), and the objective response rate was 50% (67). Conversely, Naidoo et al., in their review of 49 patients with pathologically confirmed stage IV LCNEC, concluded that LCNEC may not respond to platinum/etoposide as robustly as reported cases with extensive stage SCLC (14). In this study, the patients were identified by a search of an institutional electronic database, and available tissue was subjected to pathology re-review to confirm the diagnosis of LCNEC. There are a few prospective studies on the efficacy of platinum/etoposide doublet chemotherapy for LCNEC. Prospective, multicenter, single-arm, phase II trial of platinum/etoposide doublet chemotherapy in 42 patients with stage IIIB or IV LCNEC reported partial response in 34% of 29 patients in whom the diagnosis of LCNEC was confirmed by the centralized pathology review, and concluded that the outcome of advanced LCNEC treated with platinum/etoposide doublet chemotherapy was similar to that reported in extensive SCLC (16). Conversely, Niho et al., in their prospective, multicenter, single-arm, phase II study in patients with stage IIIB or IV LCNEC, showed that combined chemotherapy with irinotecan and platinum in patients with advanced LCNEC resulted in inferior overall survival than those with SCLC (15). In this study, the response rate was 47% for patients with LCNEC and 80% for patients with SCLC (P=0.082). The median survival time was 12.6 months in the LCNEC group and 17.3 months in the SCLC group (P=0.047). Of note, in these two prospective studies, the pathological diagnosis was confirmed by independent review by the pathology panel members followed by consensus diagnosis, and one-fourth of the cases with the original diagnosis of LCNEC were reclassified, elucidating the difficulty in the histologic classification of high-grade neuroendocrine carcinomas (15,16). Interestingly, in the aforementioned study by Rekhtman et al., three of four patients with SCLC-like LCNEC responded to platinum based cytotoxic chemotherapy, while none of six patients with NSCLC-like LCNEC (19). These lines of evidence confirm the heterogeneous morphology and biology of LCNEC.
Conclusions
Growing evidence suggests that LCNEC is a morphologically and biologically heterogeneous group of high-grade neuroendocrine carcinomas. There are morphological borderline cases between SCLC and LCNEC leading to the difficulty in classifying some high-grade neuroendocrine carcinomas into the two entities. Among these borderline tumors, some harbor molecular alterations similar to those of SCLC and others to LCNEC. Furthermore, recent molecular studies have revealed a few distinct subsets of LCNEC. Of those, the SCLC-like subset appears to exhibit SCLC-like morphology and respond to chemotherapeutic regimens for SCLC, while the NSCLC-like subset does not. The findings may explain the conflicting reports on the sensitivity of LCNEC to the SCLC regimens. Additional large cohort studies on the biology of high-grade neuroendocrine carcinomas are warranted and will aid establishing novel approaches to clinical managements of patients with LCNEC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Gosney JR, Williams IJ, Dodson AR, et al. Morphology and antigen expression profile of pulmonary neuroendocrine cells in reactive proliferations and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). Histopathology 2011;59:751-62. [Crossref] [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [Crossref] [PubMed]
- Marchevsky AM, Walts AE. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). Semin Diagn Pathol 2015;32:438-44. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol 2006;24:4526-7. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Toyozaki T, et al. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001;91:1992-2000. [Crossref] [PubMed]
- Takei H, Asamura H, Maeshima A, et al. Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc Surg 2002;124:285-92. [Crossref] [PubMed]
- Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006;24:70-6. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Baba M, et al. Pulmonary large cell carcinomas with neuroendocrine features are high-grade neuroendocrine tumors. Ann Thorac Surg 2002;73:1049-54. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prognostic impact of large cell neuroendocrine histology in patients with pathologic stage Ia pulmonary non-small cell carcinoma. J Thorac Cardiovasc Surg 2006;132:312-5. [Crossref] [PubMed]
- Naidoo J, Santos-Zabala ML, Iyriboz T, et al. Large Cell Neuroendocrine Carcinoma of the Lung: Clinico-Pathologic Features, Treatment, and Outcomes. Clin Lung Cancer 2016;17:e121-9. [Crossref] [PubMed]
- Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol 2013;8:980-4. [Crossref] [PubMed]
- Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol 2013;24:1548-52. [Crossref] [PubMed]
- Shimada Y, Niho S, Ishii G, et al. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens. Lung Cancer 2012;75:368-73. [Crossref] [PubMed]
- Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. [Crossref] [PubMed]
- Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res 2016;22:3618-29. [Crossref] [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Abstract 1531: Cross-entity mutation analysis of lung neuroendocrine tumors sheds light into their molecular origin and identifies new therapeutic targets. San Diego: AACR Annual Meeting, 2014.
- Brambilla E, Beasley MB, Chirieac LR, et al. Large cell neuroendocrine carcinoma. In: Travis WD, E. B, Burke AP et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC Press, 2015:69-72.
- Marchevsky AM, Gal AA, Shah S, et al. Morphometry confirms the presence of considerable nuclear size overlap between "small cells" and "large cells" in high-grade pulmonary neuroendocrine neoplasms. Am J Clin Pathol 2001;116:466-72. [Crossref] [PubMed]
- den Bakker MA, Willemsen S, Grunberg K, et al. Small cell carcinoma of the lung and large cell neuroendocrine carcinoma interobserver variability. Histopathology 2010;56:356-63. [Crossref] [PubMed]
- Thunnissen E, Kerr KM, Herth FJ, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung Cancer 2012;76:1-18. [Crossref] [PubMed]
- Lantuejoul S, Moro D, Michalides RJ, et al. Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol 1998;22:1267-76. [Crossref] [PubMed]
- Rossi G, Mengoli MC, Cavazza A, et al. Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Arch 2014;464:61-8. [Crossref] [PubMed]
- Travis WD, Colby TV, Corrin B, et al. World Health Organization. Histological typing of lung and pleural tumours. 3rd ed. Berlin: Springer-Verlag, 1999.
- Brambilla E, Beasley MB, Austin JH, et al. Small cell carcinoma. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC Press, 2015:63-8.
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- Travis WD, Gal AA, Colby TV, et al. Reproducibility of neuroendocrine lung tumor classification. Hum Pathol 1998;29:272-9. [Crossref] [PubMed]
- Hiroshima K. Small cell carcinoma: distinction from large cell neuroendocrine carcinoma. In: Maldonado JG, Cervantes MK. editors. Small Cell Carcinomas: Causes, Diagnosis and Treatment. New York: Nova Science Publishers, 2009:101-21.
- Pelosi G, Hiroshima K, Mino-Kenudson M. Controversial issues and new discoveries in lung neuroendocrine tumors. Diagn Histopathol 2014;20:392-7. [Crossref]
- Hiroshima K, Iyoda A, Shida T, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol 2006;19:1358-68. [Crossref] [PubMed]
- Beasley MB, Brambilla E, Chirieac LR, et al. Carcinoid tumour. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC Press, 2015:73-7.
- den Bakker MA, Thunnissen FB. Neuroendocrine tumours--challenges in the diagnosis and classification of pulmonary neuroendocrine tumours. J Clin Pathol 2013;66:862-9. [Crossref] [PubMed]
- Quinn AM, Chaturvedi A, Nonaka D. High-grade Neuroendocrine Carcinoma of the Lung With Carcinoid Morphology: A Study of 12 Cases. Am J Surg Pathol 2017;41:263-70. [Crossref] [PubMed]
- Huang Q, Muzitansky A, Mark EJ. Pulmonary neuroendocrine carcinomas. A review of 234 cases and a statistical analysis of 50 cases treated at one institution using a simple clinicopathologic classification. Arch Pathol Lab Med 2002;126:545-53. [PubMed]
- Tsuta K, Raso MG, Kalhor N, et al. Histologic features of low- and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer 2011;71:34-41. [Crossref] [PubMed]
- Ullmann R, Petzmann S, Sharma A, et al. Chromosomal aberrations in a series of large-cell neuroendocrine carcinomas: unexpected divergence from small-cell carcinoma of the lung. Hum Pathol 2001;32:1059-63. [Crossref] [PubMed]
- Hiroshima K, Iyoda A, Shibuya K, et al. Genetic alterations in early-stage pulmonary large cell neuroendocrine carcinoma. Cancer 2004;100:1190-8. [Crossref] [PubMed]
- Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999;85:600-7. [Crossref] [PubMed]
- Miyoshi T, Umemura S, Matsumura Y, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin Cancer Res 2017;23:757-65. [Crossref] [PubMed]
- Karlsson A, Brunnstrom H, Lindquist KE, et al. Mutational and gene fusion analyses of primary large cell and large cell neuroendocrine lung cancer. Oncotarget 2015;6:22028-37. [Crossref] [PubMed]
- Meder L, Konig K, Ozretic L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. [Crossref] [PubMed]
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [Crossref] [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [Crossref] [PubMed]
- Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Carretero J, Medina PP, Pio R, et al. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene 2004;23:4037-40. [Crossref] [PubMed]
- Lou G, Yu X, Song Z. Molecular Profiling and Survival of Completely Resected Primary Pulmonary Neuroendocrine Carcinoma. Clin Lung Cancer 2017;18:e197-201. [Crossref] [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [Crossref] [PubMed]
- Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892-6. [Crossref] [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [Crossref] [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998;17:475-9. [Crossref] [PubMed]
- Vollbrecht C, Werner R, Walter RF, et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: a comparison of a neglected tumour group. Br J Cancer 2015;113:1704-11. [Crossref] [PubMed]
- Kelley MJ, Nakagawa K, Steinberg SM, et al. Differential inactivation of CDKN2 and Rb protein in non-small-cell and small-cell lung cancer cell lines. J Natl Cancer Inst 1995;87:756-61. [Crossref] [PubMed]
- Washimi O, Nagatake M, Osada H, et al. In vivo occurrence of p16 (MTS1) and p15 (MTS2) alterations preferentially in non-small cell lung cancers. Cancer Res 1995;55:514-7. [PubMed]
- Girard L, Zochbauer-Muller S, Virmani AK, et al. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res 2000;60:4894-906. [PubMed]
- Jones MH, Virtanen C, Honjoh D, et al. Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet 2004;363:775-81. [Crossref] [PubMed]
- Clinical Lung Cancer Genome Project (CLCGP), Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [PubMed]
- Gugger M, Burckhardt E, Kappeler A, et al. Quantitative expansion of structural genomic alterations in the spectrum of neuroendocrine lung carcinomas. J Pathol 2002;196:408-15. [Crossref] [PubMed]
- Virmani AK, Fong KM, Kodagoda D, et al. Allelotyping demonstrates common and distinct patterns of chromosomal loss in human lung cancer types. Genes Chromosomes Cancer 1998;21:308-19. [Crossref] [PubMed]
- Bari MF, Brown H, Nicholson AG, et al. BAI3, CDX2 and VIL1: a panel of three antibodies to distinguish small cell from large cell neuroendocrine lung carcinomas. Histopathology 2014;64:547-56. [Crossref] [PubMed]
- Dresler CM, Ritter JH, Patterson GA, et al. Clinical-pathologic analysis of 40 patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg 1997;63:180-5. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Toyozaki T, et al. Adjuvant chemotherapy for large cell carcinoma with neuroendocrine features. Cancer 2001;92:1108-12. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. [Crossref] [PubMed]
- Fournel L, Falcoz PE, Alifano M, et al. Surgical management of pulmonary large cell neuroendocrine carcinomas: a 10-year experience. Eur J Cardiothorac Surg 2013;43:111-4. [Crossref] [PubMed]
- Yamazaki S, Sekine I, Matsuno Y, et al. Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin-based chemotherapy. Lung Cancer 2005;49:217-23. [Crossref] [PubMed]


