Prognostic and predictive biomarkers in early stage NSCLC: CTCs and serum/plasma markers
Introduction
Lung cancer is the most common cause of cancer related mortality in the world, responsible for 1.4 million deaths/year (1). A striking feature of lung cancer is its poor survival, with 5-year survival less than 10% in the UK and less than 15% in the USA. One major contributing factor to poor survival is the late clinical presentation of the majority of patients, 80% present with locally advanced or distant metastatic disease at which stage treatments are generally much less effective. The benefit of early diagnosis was underlined by the National Lung Screening Trial which investigated the effect of low dose CT screening in an at risk population and reported a 20% reduction in lung cancer specific mortality (2). However, achieving long-term survival even after curative intent surgery is a major challenge with recurrence occurring in 50% of cases overall and five-year survival rates of 58% to 73% for stage I, 36% to 46% for stage II and 24% for stage IIIA reported (3). Recurrence most commonly occurs at distant sites indicating the presence of micro-metastatic disease undetected by current staging strategies (4,5). Trials of platinum based adjuvant chemotherapy to treat micro-metastases have shown increases in 5-year survival in the order of 5% in patients with stage II-IIIA disease (6). The adjuvant treatment of stage I is more controversial as definitive evidence of efficacy is lacking (7). There is therefore a pressing clinical need to develop both more effective adjuvant therapy but also target the current therapies in a more effective manner. Pathological stage is the most robust methodology of selecting patients for adjuvant chemotherapy however recurrence rates even in stage I disease are in the order of 25% to 40% suggesting that an additional marker/markers that enable accurate stratification of recurrence risk over and above that provided by pathological stage are necessary for more accurate prognostication. In such a way it may be possible to stratify high-risk stage I patients who may benefit from adjuvant chemotherapy and low risk stage II who may avoid chemotherapy. Furthermore, lung cancer is a heterogenous disease and the molecular profile and/or biological behavior of disease recurring after surgery may be different to that of the primary tumor. Consequently, there may be added benefit of examining circulating factors, which may reflect the behavior and molecular profile of metastatic disease more accurately than primary tumor sampling, which could result in sampling error because of tumor heterogeneity.
Method
A literature search was performed using PubMed/Medline. Search limits set included human studies, 1990 to the present day (June 2013) and articles written in English. Initial searches using the terms: circulating biomarkers, non-small cell lung cancer (NSCLC) and prognostic/predictive were followed by more targeted searches. For circulating tumor cells (CTCs) additional search terms included: CTCs, CellSearch (CS), ISET and pulmonary vein. Nucleic acid searches used additionally: circulating nucleic acids, or DNA, or RNA or microRNA and protein searches included specific proteins including: carcinoembryonic antigen (CEA), or CYFRA 21-1, or neuron specific enolase and other identified proteins. Finally reference lists were screened for additional studies.
Results
Standard clinical measures in blood
Standard biochemical and hematological measures, taken routinely as part of the assessment for radical treatment, may in themselves provide additional prognostic information. The results of several trials are discussed below.
Hemoglobin
Tomita et al. studied 240 patients who underwent surgical resection of NSCLC (8). Classification of low hemoglobin level was <13.0 g/dL in men and <12.0 g/dL in women. Overall, 88 patients (36.7%) were classified as having a low pre-operative hemoglobin. Five-year survival was significantly lower in patients with a low hemoglobin (43.0% vs. 73.5%; P<0.0001). After multivariate analysis pre-operative hemoglobin level remained a significant poor prognostic factor.
White blood cells
The impact of peripheral pre-operative white blood cell count has been investigated in several reports (9-12). A total white blood cell count above the median has been reported to be a poor prognostic factor (9). Kobayashi et al. examined the outcomes of 237 patients with resected node negative NSCLC (10). Raised neutrophil count and low lymphocyte count were associated with survival after univariate analysis; however only a low lymphocyte count remained an indicator of poor prognosis after multivariate analysis. Similarly Zhang et al. reported elevation of peripheral lymphocytes to be a favorable prognostic factor (11). Sarraf et al. examined the ratio of neutrophil and lymphocyte counts as a prognostic marker. This measure was significantly associated with stage, however after multivariate analysis the ratio remained an independent prognostic factor (12).
Platelets
A study of 510 patients by Yu et al. examined pre-operative platelet count and outcomes in patients newly diagnosed with NSCLC (13). All patients were treated with surgical resection [clinical stage I n=234 (45.9%), stage II n=128 (25.1%) and stage III n=148 (29.0%)]. Three-year overall survival (OS) was 75.3% for patients with a normal count (≤300×109/L, n=449; 88.0%) and 59.2% for those with an elevated count (>300×109/L, n=61; 12.0%); the risk of disease progression was also increased (HR 1.57, 1.02-2.45). Multivariate analysis showed age and platelet count to be the only independent prognostic markers. Tomita et al. also reported that pre-operative thrombocytosis (>40×104/mm3) was a poor prognostic marker in a study of 289 patients undergoing resection for NSCLC. Five-year survival was 30.8% in patients with elevated platelets (n=13/289) compared with 68.7% in patients with a normal count (276/289) (14).
These studies suggest that routinely measured clinical parameters in blood may provide prognostic information. The advantages of this approach are that no additional cost is required for the assays and the assays themselves are robust. The disadvantages are that measured changes are not necessarily specific to the cancer but may be reflective of other co-morbidities or co-existing acute illnesses such as infection.
Circulating proteins
Historically circulating proteins have been most intensely investigated as prognostic biomarkers in the early lung cancer setting. The most commonly investigated proteins are CEA and CYFRA 21-1 and these studies are reviewed below.
CEA
CEA is a cell adhesion glycoprotein that is expressed in a limited number of tissues and at very low levels in healthy individuals (15). A large number of studies over the past two decades have examined the prognostic value of CEA in serum/plasma in early stage NSCLC [reviewed (16) and summarized in Table 1]. A majority of studies have found elevated levels of CEA to be associated with poor prognosis in resected NSCLC including stage I (17-21,23,27,28,30,32-35,37,40-47). A limited number of studies have reported no association (25,29,31,38,48,49). The largest study by Okada et al. examined pre and post-operative serum CEA levels in 1,000 patients undergoing resection for NSCLC (33). Patients with elevated levels of pre-operative CEA (368/1,000) had significantly lower 5-year survival compared to patients with normal levels (53.8% vs. 75.2%; P<0.0001). In patients with resected pathological stage I disease, those with persistently elevated post-operative CEA levels had significantly worse 5-year survival (48.6%) than patients whose CEA level normalized post-operatively (74.2%) and those who had normal pre-operative levels (84.2%) (34). Several studies have confirmed the association with persistently elevated CEA in the post-operative period and marked reduction in survival (17,18,30,33,39). As an example, Kosu et al. retrospectively analyzed pre and post plasma CEA levels in 263 patients with resected pathological stage I NSCLC (18). A majority had adenocarcinoma and half the population had less than 5 pack years smoking history. Patients with normal CEA before and after surgery had a 5-year survival of 95.5% compared with 85.5% (4-year survival) in patients with a pre-operative high CEA that normalized post-operatively and 59.3% in patients whose CEA was elevated both pre and post-operatively. After multivariate analysis tumor diameter greater than 30 mm, the presence of visceral pleural invasion and persistent CEA elevation were independent poor prognostic indicators. The authors conclude that patients with high post-operative CEA may benefit from adjuvant chemotherapy.
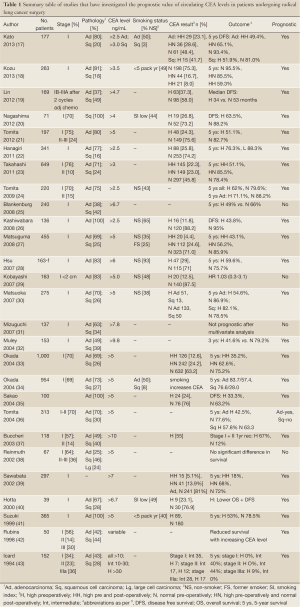
Full Table
One important aspect of many of the studies investigating CEA as a prognostic marker is that the prevalence of smoking is relatively low with upwards of half the lung cancer cases being diagnosed in never smokers. Significantly smoking may increase CEA expression in lung tissue (50). In the study by Okada et al. CEA measurement was not prognostic in current smokers potentially reducing the clinical use of CEA in populations with a higher burden of smoking induced lung carcinogenesis (34). In a majority of studies it is also unclear what role co-existing inflammatory conditions such as chronic obstructive pulmonary disease may have in modifying CEA measurements.
CYFRA 21-1 (serum cytokeratin 19 fragment)
Cytokeratins are filamentous proteins ubiquitously expressed by epithelial cells which can be used as markers of epithelial origin (51). Cytokeratin 7, 8, 18 and 19 are the most commonly seen types associated with carcinomas and these proteins can leak into the circulation and be detected as degraded complexes and as such may act as circulating biomarkers of malignancy (52,53). Degradation fragments of cytokeratin 19 can be detected in the circulation, referred to as CYFRA 21-1, released from tumor cells by necrosis or cell lysis. This protein fragment has been assessed as a prognostic marker in several studies (25,32,38,45,46,54-58). A meta-analysis of 2,063 patients with newly diagnosed NSCLC showed an elevated pre-treatment CYFRA 21-1 fragment level (>3.6 ng/mL) to be an independent poor prognostic factor at 12 months (HR 1.88, 1.64-2.15) (56). Raised CYFRA 21-1 was of borderline significance in a subpopulation of patients treated with surgical resection (survival HR 1.41, 0.99-2.03; n=437). In a study of 85 patients with squamous cell carcinoma (stages I-IIIA), the risk of death after five years of follow up was doubled in patients with CYFRA 21-1 levels above 3.6 ng/mL (HR 2.05; 1.09-3.83) (54).
Several studies have examined the combined prognostic value of pretreatment CEA and CYFRA 21-1 as a ‘tumor marker index’ (TMI) (25,32,57). Blankenburg et al. reported no prognostic value of either single or combined measures in a study of 240 patients. In contrast, studies by Tomita (57) and Muley (32) both reported improved prognostic value to the combined data. Muley et al. reported reduced 3-year survival in 153 stage I patients with raised CYFRA 21-1 baseline levels prior to surgical resection (3-year survival: high 60.2% vs. normal 78.4%). Patients were categorized according to high, intermediate and low levels of geometric mean CEA and CYFRA 21-1 and 3-year survival analyzed. Survival was 96.7% in patients with low levels, 77.2% in the intermediate group and 55.7% in patients with high levels. Tomita et al. reported reduced 5-year survival in patients with elevated pre-treatment CYFRA 21-1 (5-year survival: high 40% vs. normal 67%), in a cohort of 291 patients of stage I-III. An analysis of 5-year survival by TMI, defined as positive by the presence of elevated CYFRA 21-1 and or CEA, showed reduced survival with TIM positive patients (37.1%) compared to TMI negative patients (72.3%).
A study by Mizuguchi et al. measured Sialyl Lewisx (an important cell surface carbohydrate antigen) in addition to CYFRA 21-1 and CEA pre-operatively in 137 patients with completely resected stage I NSCLC (31). Thirty of the patients recurred and after multivariate analysis pre-operative CYFRA 21-1 and SXL levels, but not CEA, were independent prognostic markers. Patients with high levels of both markers were five times more likely to recur than those with normal values (Table 2).
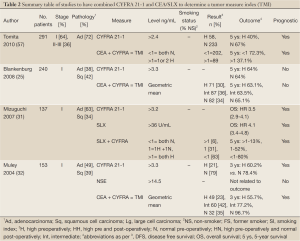
Full Table
Neuron specific enolase
Enolases are integral to the glycolysis pathway by linking 2-phosphoglycerate and phosphor-enolpyruvate metabolism. Neuron specific enolase has been associated with small cell lung cancer as a clinical biomarker (59). However several studies have examined NSE as a circulating biomarker in NSCLC (38,49,54,55,60,61). In three of the studies NSE was not prognostic (38,49), including a study of 164 patients with resected stage I disease (60). Two studies reported an association with poor prognosis in advanced disease (54,55). However, the largest study to date by Yu et al. looked at 481 patients with operable NSCLC and measured pre-operative serum levels of NSE, SCC and carbohydrate antigen 125 (CA125) (61). Both elevated levels of NSE and CA125 were associated with reduced disease free survival (DFS) and OS. In a multivariable Cox regression model advanced clinical stage and both elevation of CA125 and NSE were independent prognostic factors associated with reduced survival. Elevation of more than one circulating biomarker was associated with a worse outcome.
C-reactive protein (CRP)
Elevated CRP has been associated with poorer outcomes after surgery in two studies (62,63). O’Dowd et al. reported elevated CRP but not total white cell count to be an independent prognostic factor after multivariate analysis (63). Median survival was 75.9 months in the normal CRP group and 26.2 months in the high CRP group. Similarly Hara et al. showed reduction in 5-year disease specific (56.2% vs. 77.6%; P=0.003) and OS (50.2% vs. 74.2%; P=0.001) in the high CRP arm.
Fibrinogen
Sheng et al. report the prognostic significance of fibrinogen, an important protein in the clotting cascade, in a study of patients with operable NSCLC (n=567; 69.3% were stage I + II) (64). Normal serum fibrinogen levels (<4 g/L) were recorded in 343 patients (60.4%) compared with 224 patients (39.5%) with elevated fibrinogen levels (>4 g/L). Patients with higher baseline fibrinogen levels had lower 3-year progression-free survival (49.2% vs. 63.3%) and lower OS rates (66.0% vs. 80.9%) than patients with normal serum fibrinogen concentrations. Fibrinogen level was an independent poor prognostic marker after Cox proportional regression analysis.
Other circulating proteins
Squamous cell carcinoma antigen has been investigated in several studies without showing prognostic significance (31,54,60,65). CA125 is a standard clinical measure used in the diagnosis and monitoring of patients with ovarian cancer, but its prognostic potential has also been investigated in a small number of studies looking at patients undergoing curative resection of NSCLC. Three studies have reported elevation of pre-operative CA125 to be associated with a worse prognosis (65-67) with one study reporting no association (60).
Despite the multiple studies of circulating proteins described above none have proved sufficiently robust to incorporate into routine practice either to alter the intensity of follow up or direct adjuvant therapy.
Circulating nucleic acids
Circulating DNA can be detected in healthy individuals and in significantly higher concentrations in patients with cancer (68). Tumor specific RNA and DNA can enter the circulation through processes including necrosis and apoptosis from both primary and metastatic sites and may also be detected in plasma/serum (69).
Total circulating free DNA
Total circulating plasma DNA concentration was investigated as a potential biomarker in a population of 1,035 heavy smokers over the age of 50 who were taking part in an annual low dose CT screening study (70). A total of 38 patients were diagnosed with lung cancer during the study period. Study participation also included annual blood tests and no difference in median DNA concentration was seen between cases and controls at baseline. However, in 33 lung cancer cases who went on to have surgical resection, and who also had repeat blood samples, median DNA concentration was significantly higher within the 12-month period immediately prior to diagnosis and surgery (4.6 ng/mL) compared with samples taken over a year prior to diagnosis (2.4 ng/mL). This finding suggests that longitudinal changes in DNA concentration may predict for the development of malignancy in smokers rather than a fixed threshold concentration. In addition, five-year survival was noted to be significantly worse in patients with DNA concentrations in the highest tertile (33%) when compared to the lowest tertile. A similar association with outcome was reported by van der Drift et al., in a study of 46 patients with newly diagnosed NSCLC (29/46 were stage I to III). Significantly reduced survival was seen in patients with DNA concentrations in the highest tertile (median survival 11.8 months) compared to the lowest tertile (median survival 21.5 months) (71).
Conversely, baseline circulating free DNA concentration was not correlated with prognosis, either DFS or OS, in a study of 76 patients undergoing curative resection (stage I-II n=60, stage III n=16) (72). However in patients who relapsed early (within 3 months; n=9) increasing circulating DNA concentration was seen in a repeat blood sample taken 3 months post-operatively. By comparison patients who did not relapse showed a reduction in DNA concentration by the same time point. A similar finding was reported by Szpechcinski et al. who demonstrated reduction (n=11) or stability (n=7) in circulating DNA levels in patients who had undergone resection of NSCLC between 3 to 6 months post-surgery except for 2 individuals with early relapse whose DNA concentrations increased significantly (73). On this basis cfDNA might have an application to monitor patients for relapse prior to symptom development or radiological change and thereby inform on the intensity of follow up.
Circulating microRNAs
MicroRNAs are small non-coding single stranded RNA molecules that serve a regulatory purpose in controlling the function of messenger RNA (74,75). Tissue expression of microRNAs has been shown to be prognostic in NSCLC (76). MicroRNAs can also be detected in the circulation, where they are stable (77), and several studies have examined their prognostic significance in early stage lung cancer. Hu et al. developed a panel of 4 miRNAs (high miR-486 and miR30d; low miR1 and miR499) whose differential expression was associated with poor survival (78). Testing of the panel in 243 patients with stage I-IIIa NSCLC treated with both surgery and adjuvant chemotherapy showed the miRNAs to be an independent predictor of survival. Indeed the authors reported a dose effect dependent on how many markers in the panel were affected and increasing risk of cancer death (2 measures: HR 3.14, 1.7-6.0; 3 measures HR 16.5, 8.6-31.7; 4 measures 34.1, 16.3-71.6).
Boeri et al. examined the predictive value of miRNAs in plasma taken from two CT screening trials (79). Patients who attended for annual CT screening also had an annual blood test. A panel of 15miRNAs (mir-660, mir-140-5p, mir-451, mir-28-3p, mir-30c, and mir-92a most commonly deregulated) was able to correctly categorize 30 out of 35 patients who developed lung cancer, using plasma taken over 12 months and upwards of 28 months prior to diagnosis. Misclassification occurred in 1 out of 5 control pools. A further signature was developed (containing mir-486-5p) to explore the use of miRNA expression and prognosis at the time of diagnosis. The panel correctly classified 9 out of 11 patients with poor prognosis but misclassified 2 out of 10 patients with good prognosis.
Chen et al. examined the prognostic significance of a specific miRNA (miR-17-5p) in the serum of 221 patients newly diagnosed with lung cancer (80). Aberant expression of miR-17-5p has previously been demonstrated in lung cancer and serum miR-17-5p levels were significantly higher in this study than normal controls. Patients were stratified according to expression levels (stage I-III: high expression n=99 and low expression n=109) and survival compared. High expression was associated with a lower median survival of 33 months compared with 40 months. This difference remained after a Cox proportional hazard model (HR 1.8, 1.04-3.01). Reduced survival in patients with stage I disease (n=180) was also reported by Heegaard et al. when the population was stratified according to miR-233 levels (reduced levels associated with worse survival) (81). Silva et al. demonstrated a reduction in DFS for patients undergoing surgery (n=37) who had low levels of miR-30e-3p (DFS rate at 50 months =13%, 14-52) compared with high levels (DFS rate 50%, 23-77) (82). Measures of plasma let-7f and miR-20b were not associated with DFS. Survival was associated with differential expression of three miRNAs (miR-96, miR-182, and miR-183) in a study of 70 patients with NSCLC treated with surgical resection (76).
Circulating mRNA
Cheng et al. explored the prognostic value of relative levels of circulating cMET mRNA in blood from 45 patients undergoing resection using RT-PCR (83). Over-expression of cMET, a proto-oncogene implicated in angiogenesis and metastasis, was recorded in 23 patients and after a mean follow up period of 23 months 18 (78.3%) of these patients had recurred and 8 died. This compared with 4 (18.2%) recurrences and no deaths in the cMET negative group. After multivariate analysis cMET positivity was the strongest predictor of recurrence (HR 3.9, 1.2-13.3). Hepatocyte growth factor (HGF), the ligand for cMET, has been variably associated with outcome in early stage NSCLC; reported to be prognostic in studies with small samples sizes (84,85) but not in a much larger study of 196 patients (86).
CTCs
CTCs or Circulating Tumor Microemboli (CTMs, cells in contiguous groups) are postulated to have a critical role in the development of metastatic disease (87). The detection of CTCs in patients with lung cancer has been described in numerous clinical studies and has in general been associated with a poorer prognosis (88). A number of different methods have been used [reviewed (89)] and the field is rapidly evolving; current methodologies can be broadly divided into assays that physically isolate individual cells/groups of cells for further characterization and nucleic acid based techniques, which infer the presence of CTCs.
Cell based detection
The most robustly developed platform is CellSearch (CS), which is fully validated and approved for clinical use in advanced breast, bowel and prostate cancer. CTC enrichment from blood is performed automatically (CellTracks AutoPrep) through immunomagnetic selection of epithelial cell adhesion molecule (EpCAM) expressing cells, using ferrofluid particles coated with an anti-EpCAM antibody, which are then separated from blood by magnets. Selected cells are then stained with phycoerythrin-conjugated anti-cytokeratin (8,40,44) antibodies (epithelial cell marker), allophycocyanin-conjugated anti-CD45 antibodies (white blood cell marker) and a nuclear stain (4',6-diamidino-2-phenylindole; DAPI). Analysis is undertaken using a semi-automatic fluorescence microscope (CellTracks Analyser II) and CTCs categorised using morphological (round or oval, visible nucleus in cytoplasm, cells at least 4 µm in diameter) and immunofluorescent criteria (CK+, DAPI+ and CD45–). The automated processing and semi-automated analysis produce low inter and intra-assay variability (90). Using this method, our group has shown that CTC number is an independent prognostic marker in advanced NSCLC; in univariate analysis, patients ≥5 CTCs had an OS of 4.3 months compared with 8.1 months in those with <5 CTCs (P<0.001). Indeed, CTC number was the strongest predictor of OS (HR 7.9, 2.9-22.0; P<0.001) after multivariate analysis. However, ≥2 CTCs were detected in only 32% of stage IV patients (19/60) and rarely in patients with stage IIIB (7%, 2/27) and not at all in stage IIIA (0%, 0/14) (91). One possible disadvantage of the CS approach is the reliance on EpCAM positive selection. EpCAM expression is commonly found in tumours of epithelial origin (92), but not necessarily on all CTCs (93).
The theory of epithelial to mesenchymal transition proposes that cells develop a more metastatic phenotype, reflected by down regulation of epithelial markers and upregulation of mesenchymal markers, that facilitates migration from the primary tumour into the circulation (94). Selection of CTCs based purely on epithelial markers may underestimate the CTC burden and therefore lower the sensitivity of this approach; this may be especially relevant in NSCLC (95). Selection of CTCs using non-epithelial based markers may therefore have advantages; one example is ISET (Isolation by Size of Epithelial Tumour cells) (96). ISET technology involves the filtering of blood through a membrane with 8 µm pores to isolate cells or groups of cells larger than this size independently of cellular protein expression. Cells may then be characterized morphologically and by protein expression. Using this method in a direct comparison with CS we have shown that ISET identifies significantly more CTCs and CTMs in a study of 40 patients with advanced lung cancer (97). In this study ISET demonstrated CTCs in 80% (32/40) of patients compared with 23% (9/40) using CS; CTCs were detected by both methods in only 7 patients and although CTMs were detected in 38% (15/50) by ISET, CS identified no CTMs.
In the early non-small cell cancer setting, Hofman et al. investigated the prognostic value of CTCs detected in peripheral blood drawn from 210 patients prior to surgical resection of NSCLC using both CS and ISET (98,99). Pathological stage was I or II in 62% (131/210) and III or IV in the remainder (38%, 79/210). CTCs were standardly defined (CK+, DAPI+, CD45–) with CS and with ISET as cells with positive immunocytochemical staining for cytokeratin and/or vimentin with morphological features of non-hematological cells. CTCs were detected in 69% of patients overall [ISET 50% + ve (104/210); CS + ve: ≥1 in 39% (82/210) and ≥2 in 21% (44/210)]. CTC number was not related to disease stage or histological subtype. CTC counts were higher using ISET (mean 34, range, 1 to 23) compared with CS (mean 12, range, 1 to 150). In the 144 patients where CTCs were detected only one in five (20%, 42/210) were detected using both CS and ISET. ISET cells stained with cytokeratin alone in 26.0% (27/104), cytokeratin and vimentin in 52.9% (55/104) and vimentin alone in 22.1% (23/104). Multivariate Cox proportional hazard regression analysis showed the presence of CTCs to be a poor prognostic factor, associated with reduced DFS after a median follow up of 15 months, irrespective of method used for CTC detection (CS: HR 1.6, 1.3-4.7; P=0.008. ISET: HR 1.4, 1.1-3.3; P=0.006).
A small study by Sawabata et al. examined CTC count using CS in peripheral blood immediately pre and post operatively and 10 days after surgical resection of NSCLC. CTCs were detected in 1 of 9 before, 3 out of nine patients immediately after and in no patients 10 days after surgery. After a median follow up of 14 months no patient had evidence of relapse (100).
Nucleic acid based detection
Nucleic acid based methodologies to detect CTCs have been used in the early NSCLC setting. A study by Yie et al. applied a RT-PCR ELISA technique to the cellular fraction of peripheral blood (2 mL) taken from patients with NSCLC to determine the concentration of survivin mRNA (101). Survivin is a protein commonly overexpressed in malignancy and has a role in tumor progression (102), a recent meta-analysis reported expression of survivin in NSCLC to be a poor prognostic factor (103). The authors determined an upper limit of normal for survivin mRNA concentration in blood by examining 172 healthy volunteers. Levels above the highest in this cohort were then classed as abnormal and designated as CTC positive. Using this classification, 44.1% of patients with NSCLC taking part in the study had detectable CTCs (n=63/143), corresponding to 26% of stage I + II (n=13/50) and 47.9% (n=35/73) of stage III patients. Follow up for a median period of 36 months was performed on 67 patients who were treated with surgical resection; of these 26 were CTC positive (38.8%). A total of 18 relapses occurred in the CTC positive cohort (n=18/26; 60% stage I + II, 75% stage III) compared with 4 in the CTC negative cohort (n=4/41; 15.9% stage I + II, 8.3% stage III). The likelihood of relapse was significantly higher in CTC positive patients (RR 43.5, 2.7-70.9; P=0.008).
A panel of 4 marker genes (homo sapiens keratin 19, ubiquitin thiolesterase, highly similar to HSFIB1 for fibronectin, tripartite motif-containing 28) was developed by Sher et al. and tested (RT-PCR) in a cohort of 54 patients undergoing curative resection of NSCLC (104). The panel was determined by examining genes with large differential expression ratios between lung cancer cell lines and white blood cells. Detection of any of the marker genes classified a sample as CTC positive; the detection rate was 72% (39/54). The method was further developed to allow semi-quantitative measurement of the relative expression of marker genes to determine a circulating tumor cell load; which was validated using spiked tumor cell experiments. Patients were categorized into high and low cancer cell load for analysis and those with a low cancer cell load had better prognosis when matched for stage.
Yamashita et al. examined the blood of 103 patients with NSCLC pre and post-surgery (105). CTCs were defined by the detection of mRNA for CEA, which is expressed in epithelial cells. Patients with detectable CEA in the pre-operative blood sample had a significantly worse prognosis. Median survival in patients with a positive pre-operative test was 14 months compared to over 26 months in those with a negative test. Multivariate analysis showed that stage and CEA mRNA detection were independent prognostic factors (P=0.0004, RR 0.21). Yoon et al. sampled blood from patients before and after resection of NSCLC (n=79) and defined CTC status by the presence of thyroid transcription factor-1 (TTF-1) and cytokeratin19 (CK19) mRNA using real time RT-PCR (106). TTF-1 was detected in 36.1% (22/61) and 37.5% of patients before and after surgery respectively. CK19 mRNA positive samples were detected in 42.6% (26/61) and 25.0% (12/48) before and after surgery. Post-surgery positivity for both measures was most strongly correlated with shorter disease-free survival (P=0.006). Patients who only had post-surgery positive TTF-1 samples had a significantly shorter disease-free survival (P<0.001); this was not the case for CK19, which was not prognostic when measured independently.
Pulmonary vein sampling
In addition to analysis of peripheral blood, a small number of studies using various methodologies have explored the presence of CTCs within the pulmonary vein at the time of surgical resection. The pulmonary veins drain blood directly from the lungs and deliver oxygenated blood to the heart for distribution to the systemic circulation; their proximity to the primary tumor and the ability to take blood before the filtering effect of the capillary bed, which has been reported to remove 90% of CTCs (107), makes pulmonary vein sampling potentially advantageous. In a study of 30 patients undergoing resection of lung cancer, Okumura et al. detected CTCs in 96% (n=29/30) of pulmonary compared with 17% (n=5/30) of matched peripheral vein samples (using CS) (108). Pulmonary vein CTC count was not prognostic after a median follow-up period of 13 months (2 deaths from lung cancer-one patient with high and one with low pulmonary vein CTC count).
A novel method of CTC detection, involving a CD45 negative enrichment step followed by density gradient centrifugation, was developed by Funaki et al. who reported a pulmonary vein CTC prevalence of 72% (n=68/94) (109). Circulating tumor micro-emboli were detected in over half of patients with a CTC positive blood sample (n=35/68). Recurrence occurred in a total of 16 patients at local (n=4), local and distant (n=7) and distant only (n=5) sites after a median follow up period of 13 months. Of these 16 patients 15 had detectable CTCs or CTMs in the pulmonary vein at the time of surgery. Multivariate analysis showed the presence of pulmonary vein CTMs (RR 8.9, 1.7-21.0; P=0.006) and tumor stage III and IV (RR 9.8, 3.4-40.4; P=0.002) to be the only significant predictors of relapse.
Sienel et al., using an anti-cytokeratin antibody to define CTCs, detected pulmonary vein CTCs in 18% of patients (11/62) (110). There was an overall trend towards poor prognosis in patients with detectable CTCs, 7/11 died from lung cancer compared to 13/39 patients with no CTCs detected (P=0.054). Subgroup analysis of patients with no mediastinal nodal involvement (i.e., N0 or N1) showed CTC detection to be a significantly adverse prognostic marker (RR 4.2, 1.6-11.1; P=0.004) after multivariate analysis. Pulmonary vein CTCs were also shown to have adverse prognostic significance by Dong et al. using flow cytometry to detect CTCs (defined as CD45–, CK+, 2F7/S5A+) (111). Of 31 patients included in the study 15 had detectable CTCs (48.4%). Median follow up was 30 months and patients with CTCs in the pulmonary vein had significantly worse prognosis [2-year survival 62.5% (CTC – ve) vs. 26.7% (CTC + ve); P=0.023]. Multivariate analysis showed that only disease stage and CTC positive test (RR 2.8, 1.1-7.2; P=0.03) were the only independent prognostic tests. By contrast, Franco et al. reported the presence of CTCs in the pulmonary vein of 23.9% of patients undergoing resection of NSCLC (n=11/45) but CTC number was not related to prognosis (112).
In these studies the prevalence of pulmonary vein CTCs was markedly different (18% to 96%). The interpretation of these results needs to take into account not only methodological differences in CTC isolation and characterization but also the exact timing of pulmonary vein sampling with respect to the operation itself. Surgical manipulation of lungs peri-operatively has been investigated as a potential cause of increased CTCs. Two studies examining the sequence of vessel ligation at the time of surgery concluded that initial pulmonary vein ligation was associated with a lower post-operative release of CTCs than pulmonary artery ligation (113,114). However, studies by Yamashita et al. and Kozak et al. reported that the sequence of vessel ligation had no significant impact on prognosis (105,115). In three of the studies pulmonary vein sampling was performed after lung resection, potentially artificially elevating CTC numbers. Nevertheless, collectively the results are provocative for CTC analysis from pulmonary vein samples to inform on prognosis and thereby therapeutic decision making.
In summary, these studies overall have demonstrated the presence of CTCs in the surgical setting and have associated the finding of CTCs with a poorer prognosis. The utility of CTC detection has not yet been developed to a point that could guide clinical decision making and further prospective studies are required for this purpose.
Conclusions and future directions
Achieving long-term survival in patients with radically treated early stage lung cancer remains a major challenge with recurrence rates overall of 50%. Several different circulating biomarkers show promise as indicators of prognosis in patients with resected NSCLC. As an example, CEA has been studied most extensively and recent studies measuring pre and post-operative CEA levels have identified a small proportion of stage I adenocarcinoma patients with a particularly poor prognosis who may benefit from adjuvant chemotherapy. Evidence is lacking however for the predictive value of CEA in this setting. Some studies were performed prior to the routine use of adjuvant therapy or in stage I patients who are not routinely treated. In addition, the prognostic value of CEA may be more applicable to countries with a significant proportion of non-smoking lung cancer cases (up to 50% in some reports). The clinical value in European and American populations is less clear-cut, where there is a much heavier burden of smoking induced lung cancer and as a consequence common co-existence of inflammatory lung conditions such as chronic obstructive pulmonary disease.
A panel of biomarkers may be more reliable in predicting prognosis than a single measure, e.g., combined CYFRA 21-1 and CEA, which have been shown in several studies to be more strongly associated with prognosis than each individual measure taken alone. Newer markers such as circulating nucleic acids (DNA or microRNAs) or CTCs have the potential to reflect directly the biological behavior and provide molecular insights into the tumor biology itself. However, due to the ready availability and low cost of protein based markers in clinical laboratories, it would be prudent to include for example CEA or CYFRA 21-1 in any prospective study of a novel biomarker requiring more sophisticated and costly technology.
There are a multitude of studies that have examined circulating biomarkers and prognosis post-surgical resection. Results do indicate that it may be possible to differentiate patients with similar pathologies and stage into high and low risk categories based on the probability of recurrence on the basis of a convenient blood based test. Adjuvant therapy trials of the late 1990s/early 2000s did not incorporate translational studies of circulating biomarkers; focusing instead on tissue markers in resected tumor tissue. With the availability of new technologies and the opportunities they provide it may be opportune to revisit adjuvant trials with prospective evaluation of circulating biomarkers.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Choi YS, Shim YM, Kim K, et al. Pattern of recurrence after curative resection of local (stage I and II) non-small cell lung cancer: difference according to the histologic type. J Korean Med Sci 2004;19:674-6. [PubMed]
- Boyd JA, Hubbs JL, Kim DW, et al. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 2010;5:211-4. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
-
NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77.
[PubMed] - Tomita M, Shimizu T, Hara M, et al. Impact of preoperative hemoglobin level on survival of non-small cell lung cancer patients. Anticancer Res 2008;28:1947-50. [PubMed]
- Carus A, Ladekarl M, Hager H, et al. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: No immediate impact on patient outcome. Lung Cancer 2013;81:130-7. [PubMed]
- Kobayashi N, Usui S, Kikuchi S, et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 2012;75:223-7. [PubMed]
- Zhang J, Huang SH, Li H, et al. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol 2013;30:352. [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [PubMed]
- Yu D, Liu B, Zhang L, et al. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med 2013;5:1351-54. [PubMed]
- Tomita M, Shimizu T, Ayabe T, et al. Prognostic significance of the combined use of preoperative platelet count and serum carcinoembryonic antigen level in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg 2010;58:573-6. [PubMed]
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 1999;9:67-81. [PubMed]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138-43. [PubMed]
- Kato T, Ishikawa K, Aragaki M, et al. Optimal predictive value of preoperative serum carcinoembryonic antigen for surgical outcomes in stage I non-small cell lung cancer: Differences according to histology and smoking status. J Surg Oncol 2013;107:619-24. [PubMed]
- Kozu Y, Maniwa T, Takahashi S, et al. Prognostic significance of postoperative serum carcinoembryonic antigen levels in patients with completely resected pathological-stage I non-small cell lung cancer. J Cardiothorac Surg 2013;8:106. [PubMed]
- Lin XF, Wang XD, Sun DQ, et al. High serum CEA and CYFRA21-1 levels after a two-cycle adjuvant chemotherapy for NSCLC: possible poor prognostic factors. Cancer Biol Med 2012;9:270-3. [PubMed]
- Nagashima T, Sakao Y, Mun M, et al. A clinicopathological study of resected small-sized squamous cell carcinomas of the peripheral lung: prognostic significance of serum carcinoembryonic antigen levels. Ann Thorac Cardiovasc Surg 2012. [Epub ahead of print]. [PubMed]
- Tomita M, Shimizu T, Ayabe T, et al. Maximum SUV on positron emission tomography and serum CEA level as prognostic factors after curative resection for non-small cell lung cancer. Asia Pac J Clin Oncol 2012;8:244-7. [PubMed]
- Hanagiri T, Sugaya M, Takenaka M, et al. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer 2011;74:112-7. [PubMed]
- Takahashi N, Suzuki K, Takamochi K, et al. Prognosis of surgically resected lung cancer with extremely high preoperative serum carcinoembryonic antigen level. Gen Thorac Cardiovasc Surg 2011;59:699-704. [PubMed]
- Tomita M, Shimizu T, Hara M, et al. Serum carcinoembryonic antigen level in non-small-cell lung cancer patients with preoperative normal serum level. Gen Thorac Cardiovasc Surg 2009;57:303-6. [PubMed]
- Blankenburg F, Hatz R, Nagel D, et al. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer: external validation of a prognostic score. Tumour Biol 2008;29:272-7. [PubMed]
- Kashiwabara K, Saeki S, Sasaki J, et al. Combined evaluation of postoperative serum levels of carcinoembryonic antigen less than or equal to 2.5 ng/ml and absence of vascular invasion may predict no recurrence of stage I adenocarcinoma lung cancer. J Thorac Oncol 2008;3:1416-20. [PubMed]
- Matsuguma H, Nakahara R, Igarashi S, et al. Pathologic stage I non-small cell lung cancer with high levels of preoperative serum carcinoembryonic antigen: clinicopathologic characteristics and prognosis. J Thorac Cardiovasc Surg 2008;135:44-9. [PubMed]
- Hsu WH, Huang CS, Hsu HS, et al. Preoperative serum carcinoembryonic antigen level is a prognostic factor in women with early non-small-cell lung cancer. Ann Thorac Surg 2007;83:419-24. [PubMed]
- Kobayashi N, Toyooka S, Soh J, et al. Risk factors for recurrence and unfavorable prognosis in patients with stage I non-small cell lung cancer and a tumor diameter of 20 mm or less. J Thorac Oncol 2007;2:808-12. [PubMed]
- Matsuoka K, Sumitomo S, Nakashima N, et al. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:435-9. [PubMed]
- Mizuguchi S, Nishiyama N, Iwata T, et al. Serum Sialyl Lewis x and cytokeratin 19 fragment as predictive factors for recurrence in patients with stage I non-small cell lung cancer. Lung Cancer 2007;58:369-75. [PubMed]
- Muley T, Dienemann H, Ebert W. CYFRA 21-1 and CEA are independent prognostic factors in 153 operated stage I NSCLC patients. Anticancer Res 2004;24:1953-6. [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg 2004;78:216-21. [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of histologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non-small cell lung carcinoma. Ann Thorac Surg 2004;78:1004-9; discussion 1009-10. [PubMed]
- Sakao Y, Tomimitsu S, Takeda Y, et al. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 2004;25:520-2. [PubMed]
- Tomita M, Matsuzaki Y, Edagawa M, et al. Prognostic significance of preoperative serum carcinoembryonic antigen level in lung adenocarcinoma but not squamous cell carcinoma. Ann Thorac Cardiovasc Surg 2004;10:76-80. [PubMed]
- Buccheri G, Ferrigno D. Identifying patients at risk of early postoperative recurrence of lung cancer: a new use of the old CEA test. Ann Thorac Surg 2003;75:973-80. [PubMed]
- Reinmuth N, Brandt B, Semik M, et al. Prognostic impact of Cyfra21-1 and other serum markers in completely resected non-small cell lung cancer. Lung Cancer 2002;36:265-70. [PubMed]
- Sawabata N, Ohta M, Takeda S, et al. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. Ann Thorac Surg 2002;74:174-9. [PubMed]
- Hotta K, Segawa Y, Takigawa N, et al. Evaluation of the relationship between serum carcinoembryonic antigen level and treatment outcome in surgically resected clinical-stage I patients with non-small-cell lung cancer. Anticancer Res 2000;20:2177-80. [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Prognostic factors in clinical stage I non-small cell lung cancer. Ann Thorac Surg 1999;67:927-32. [PubMed]
- Rubins JB, Dunitz J, Rubins HB, et al. Serum carcinoembryonic antigen as an adjunct to preoperative staging of lung cancer. J Thorac Cardiovasc Surg 1998;116:412-6. [PubMed]
- Icard P, Regnard JF, Essomba A, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in resected primary lung cancer. Ann Thorac Surg 1994;58:811-4. [PubMed]
- Ford CH, Stokes HJ, Newman CE. Carcinoembryonic antigen and prognosis after radical surgery for lung cancer: immunocytochemical localization and serum levels. Br J Cancer 1981;44:145-53. [PubMed]
- Kao CH, Hsieh JF, Ho YJ, et al. Cytokeratin fragment 19 (CYFRA 21-1) and carcinoembryonic antigen for early prediction of recurrence of lung adenocarcinoma. Lung 1999;177:333-7. [PubMed]
- Nisman B, Amir G, Lafair J, et al. Prognostic value of CYFRA 21-1, TPS and CEA in different histologic types of non-small cell lung cancer. Anticancer Res 1999;19:3549-52. [PubMed]
- Niklinski J, Furman M, Laudanski J, et al. Prognostic value of pretreatment CEA, SCC-Ag and CA 19-9 levels in sera of patients with non-small cell lung cancer. Eur J Cancer Prev 1992;1:401-6. [PubMed]
- Buccheri G, Ferrigno D, Vola F. Carcinoembryonic antigen (CEA), tissue polypeptide antigen (TPA) and other prognostic indicators in squamous cell lung cancer. Lung Cancer 1993;10:21-33. [PubMed]
- Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer. Anticancer Res 1999;19:3613-8. [PubMed]
- Ohwada A, Takahashi H, Nagaoka I, et al. Effect of cigarette smoke on the mRNA and protein expression of carcinoembryonic antigen (CEA), a possible chemoattractant for neutrophils in human bronchioloalveolar tissues. Thorax 1995;50:651-7. [PubMed]
- Buccheri G, Ferrigno D. Lung tumor markers of cytokeratin origin: an overview. Lung Cancer 2001;34 Suppl 2:S65-9. [PubMed]
- Sundström BE, Stigbrand TI. Cytokeratins and tissue polypeptide antigen. Int J Biol Markers 1994;9:102-8. [PubMed]
- Broers JL, Ramaekers FC, Rot MK, et al. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res 1988;48:3221-9. [PubMed]
- Kulpa J, Wojcik E, Reinfuss M, et al. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA 21-1, and neuron-specific enolase in squamous cell lung cancer patients. Clin Chem 2002;48:1931-7. [PubMed]
- Pujol JL, Boher JM, Grenier J, et al. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer 2001;31:221-31. [PubMed]
- Pujol JL, Molinier O, Ebert W, et al. CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: results of a meta-analysis in 2063 patients. Br J Cancer 2004;90:2097-105. [PubMed]
- Tomita M, Shimizu T, Ayabe T, et al. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21-1 in non-small cell lung cancer. Anticancer Res 2010;30:3099-102. [PubMed]
- Bréchot JM, Chevret S, Nataf J, et al. Diagnostic and prognostic value of Cyfra 21-1 compared with other tumour markers in patients with non-small cell lung cancer: a prospective study of 116 patients. Eur J Cancer 1997;33:385-91. [PubMed]
- Pujol JL, Quantin X, Jacot W, et al. Neuroendocrine and cytokeratin serum markers as prognostic determinants of small cell lung cancer. Lung Cancer 2003;39:131-8. [PubMed]
- Ma S, Shen L, Qian N, et al. The prognostic values of CA125, CA19.9, NSE, AND SCC for stage I NSCLC are limited. Cancer Biomark 2011-2012;10:155-62. [PubMed]
- Yu D, Du K, Liu T, et al. Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients. Int J Mol Sci 2013;14:11145-56. [PubMed]
- Hara M, Matsuzaki Y, Shimuzu T, et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res 2007;27:3001-4. [PubMed]
- O’Dowd C, McRae LA, McMillan DC, et al. Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. J Thorac Oncol 2010;5:988-92. [PubMed]
- Sheng L, Luo M, Sun X, et al. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer 2013;133:2720-5. [PubMed]
- Diez M, Gomez A, Hernando F, et al. Serum CEA, CA125, and SCC antigens and tumor recurrence in resectable non-small cell lung cancer. Int J Biol Markers 1995;10:5-10. [PubMed]
- Diez M, Torres A, Pollan M, et al. Prognostic significance of serum CA 125 antigen assay in patients with non-small cell lung cancer. Cancer 1994;73:1368-76. [PubMed]
- Gaspar MJ, Diez M, Rodriguez A, et al. Clinical value of CEA and CA125 regarding relapse and metastasis in resectable non-small cell lung cancer. Anticancer Res 2003;23:3427-32. [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [PubMed]
- Bremnes RM, Sirera R, Camps C. Circulating tumour-derived DNA and RNA markers in blood: a tool for early detection, diagnostics, and follow-up? Lung Cancer 2005;49:1-12. [PubMed]
- Sozzi G, Roz L, Conte D, et al. Plasma DNA quantification in lung cancer computed tomography screening: five-year results of a prospective study. Am J Respir Crit Care Med 2009;179:69-74. [PubMed]
- van der Drift MA, Hol BE, Klaassen CH, et al. Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer 2010;68:283-7. [PubMed]
- Ludovini V, Pistola L, Gregorc V, et al. Plasma DNA, microsatellite alterations, and p53 tumor mutations are associated with disease-free survival in radically resected non-small cell lung cancer patients: a study of the perugia multidisciplinary team for thoracic oncology. J Thorac Oncol 2008;3:365-73. [PubMed]
- Szpechcinski A, Chorostowska-Wynimko J, Kupis W, et al. Quantitative analysis of free-circulating DNA in plasma of patients with resectable NSCLC. Expert Opin Biol Ther 2012;12 Suppl 1:S3-9. [PubMed]
- Mallick R, Patnaik SK, Yendamuri S. MicroRNAs and lung cancer: Biology and applications in diagnosis and prognosis. J Carcinog 2010;9:8. [PubMed]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259-69. [PubMed]
- Yu SL, Chen HY, Chang GC, et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 2008;13:48-57. [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513-8. [PubMed]
- Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 2010;28:1721-6. [PubMed]
- Boeri M, Verri C, Conte D, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A 2011;108:3713-8. [PubMed]
- Chen Q, Si Q, Xiao S, et al. Prognostic significance of serum miR-17-5p in lung cancer. Med Oncol 2013;30:353. [PubMed]
- Heegaard NH, Schetter AJ, Welsh JA, et al. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int J Cancer 2012;130:1378-86. [PubMed]
- Silva J, Garcia V, Zaballos A, et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J 2011;37:617-23. [PubMed]
- Cheng TL, Chang MY, Huang SY, et al. Overexpression of circulating c-met messenger RNA is significantly correlated with nodal stage and early recurrence in non-small cell lung cancer. Chest 2005;128:1453-60. [PubMed]
- Hosoda H, Izumi H, Tukada Y, et al. Plasma hepatocyte growth factor elevation may be associated with early metastatic disease in primary lung cancer patients. Ann Thorac Cardiovasc Surg 2012;18:1-7. [PubMed]
- Ujiie H, Tomida M, Akiyama H, et al. Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res 2012;32:3251-8. [PubMed]
- D’Amico TA, Brooks KR, Joshi MB, et al. Serum protein expression predicts recurrence in patients with early-stage lung cancer after resection. Ann Thorac Surg 2006;81:1982-7; discussion 1987.
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [PubMed]
- Ma XL, Xiao ZL, Liu L, et al. Meta-analysis of circulating tumor cells as a prognostic marker in lung cancer. Asian Pac J Cancer Prev 2012;13:1137-44. [PubMed]
- Allan AL, Keeney M. Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol 2010;2010:426218.
- Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920-8. [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [PubMed]
- Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004;35:122-8. [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [PubMed]
- Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 2011;105:847-53. [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57-63. [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [PubMed]
- Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35. [PubMed]
- Sawabata N, Okumura M, Utsumi T, et al. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg 2007;55:189-92. [PubMed]
- Yie SM, Lou B, Ye SR, et al. Clinical significance of detecting survivin-expressing circulating cancer cells in patients with non-small cell lung cancer. Lung Cancer 2009;63:284-90. [PubMed]
- Duffy MJ, O’Donovan N, Brennan DJ, et al. Survivin: a promising tumor biomarker. Cancer Lett 2007;249:49-60. [PubMed]
- Huang LN, Wang DS, Chen YQ, et al. Expression of survivin and patients survival in non-small cell lung cancer: a meta-analysis of the published studies. Mol Biol Rep 2013;40:917-24. [PubMed]
- Sher YP, Shih JY, Yang PC, et al. Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin Cancer Res 2005;11:173-9. [PubMed]
- Yamashita J, Matsuo A, Kurusu Y, et al. Preoperative evidence of circulating tumor cells by means of reverse transcriptase-polymerase chain reaction for carcinoembryonic antigen messenger RNA is an independent predictor of survival in non-small cell lung cancer: a prospective study. J Thorac Cardiovasc Surg 2002;124:299-305. [PubMed]
- Yoon SO, Kim YT, Jung KC, et al. TTF-1 mRNA-positive circulating tumor cells in the peripheral blood predict poor prognosis in surgically resected non-small cell lung cancer patients. Lung Cancer 2011;71:209-16. [PubMed]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. [PubMed]
- Okumura Y, Tanaka F, Yoneda K, et al. Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg 2009;87:1669-75. [PubMed]
- Funaki S, Sawabata N, Nakagiri T, et al. Novel approach for detection of isolated tumor cells in pulmonary vein using negative selection method: morphological classification and clinical implications. Eur J Cardiothorac Surg 2011;40:322-7. [PubMed]
- Sienel W, Seen-Hibler R, Mutschler W, et al. Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: detection rate and clinical significance. Eur J Cardiothorac Surg 2003;23:451-6. [PubMed]
- Dong Q, Huang J, Zhou Y, et al. Hematogenous dissemination of lung cancer cells during surgery: quantitative detection by flow cytometry and prognostic significance. Lung Cancer 2002;37:293-301. [PubMed]
- Franco R, Pirozzi G, Scala S, et al. CXCL12-binding receptors expression in non-small cell lung cancer relates to tumoral microvascular density and CXCR4 positive circulating tumoral cells in lung draining venous blood. Eur J Cardiothorac Surg 2012;41:368-75. [PubMed]
- Kurusu Y, Yamashita J, Hayashi N, et al. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg 1998;116:107-13. [PubMed]
- Ge MJ, Shi D, Wu QC, et al. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol 2006;132:248-56. [PubMed]
- Kozak A, Alchimowicz J, Safranow K, et al. The impact of the sequence of pulmonary vessel ligation during anatomic resection for lung cancer on long-term survival--a prospective randomized trial. Adv Med Sci 2013;58:156-63. [PubMed]


