Gefitinib in non-small-cell lung cancer—an old lesson new re-visited
Non-small-cell lung cancer (NSCLC) accounts for approximately 80-85% of all cases of lung cancer, and is the most common cause of death in men and second only to breast cancer in woman (1). Treatment of NSCLC is guided by disease stage. Surgery is the treatment of choice for early-stage localized disease, whereas multimodality therapy remains the norm for patients with locally advanced disease. Patients with advanced metastatic disease may derive a benefit from palliative chemotherapy. About 40% of patients with NSCLC present at an advanced stage, with metastatic or locally advanced disease, which underscores the importance of identifying therapeutic schemes that may benefit this large patient population. Combination chemotherapy, usually platinum-based, is currently the first-line therapy of choice (2). Based on various studies, doublet regimens containing cisplatin or carboplatin with paclitaxel, gemcitabine, docetaxel, vinorelbine or irinotectan are administered. The choice of combination drugs, however, varies in different countries, but several studies have shown similar degrees of efficacy among different combinations in the treatment of advanced NSCLC (3).
The prognosis for patients with advanced NSCLC is poor. Recent, large, randomized phase III trials have demonstrated that platinum-based chemotherapy combinations yield a median survival time of 8-11 months, a 1-year survival rate of 30-45% and a 2-year survival rate of 10-20% (4). The treatment of NSCLC is therefore a major unmet need and new therapies focusing on the molecular mechanisms that mediate the growth of lung cancer cells are urgently needed.
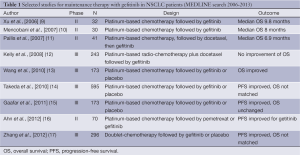
Maintenance therapy is a treatment strategy that has been investigated extensively in NSCLC and has been the subject of considerable recent debate. Options for maintenance include continuing the initial combination chemotherapy regimen, continuing only single agent chemotherapy (‘continuation maintenance’) or introducing a new agent (‘switch’ maintenance therapy). Therapies that have been studied in this setting in randomized trials to date include chemotherapy, molecularly targeted agents and immunotherapy approaches (5). Following the development of multiple new agents that show activity in NSCLC, and have a tolerable side-effect profile, there has been increasing interest in utilizing them to maintain response to initial therapy after treatment with platinum-based doublets (6). The outstanding results of the JMEN study proved that maintenance of pemetrexed (for patients with tumors of non-squamous histology) significantly improved the overall survival (OS) in advanced NSCLC patients was a proof of principle (7). Subsequently, the results of the SATURN study also showed a significant prolongation of progression-free survival (PFS) and OS with maintenance erlotinib (for patients with stable disease) compared with placebo (8). Despite considerable controversy (see Table 1), it has become an acceptable treatment paradigm and both drugs are approved for maintenance therapy of advanced NSCLC patients in Europe and the USA.
Full table
Gefitinib (Iressa®, AstraZeneca, UK) is targeted against tyrosine kinase activity on the EGF-R pathway. Gefitinib has an interesting development history and has contributed greatly to our understanding of the biology of NSCLC and the role of the EGF-R signalling pathways. During the phase II dose finding studies (IDEAL studies 1 and 2) gefitinib showed activity as monotherapy in patients with advanced NSCLC who had received prior chemotherapy with overall response rates of 19% (IDEAL 1, Asian-European trial) and 10% (IDEAL 2, US trial) (18).
Since maintenance therapy with gefitinib of patients with advanced NSCLC without disease progression after first-line chemotherapy (platinum-based) is still not established, Zhang and colleagues (17) have recently reported results of the INFORM trial. In this large phase III multicentre, double-blind trial patients (Asian ethnic origin, N=296) with stage IIIb or IV NSCLC after four cycles of platinum-based doublet chemotherapy were randomised either to placebo or maintenance therapy with gefitinib (250 mg/d) until progression or unacceptable toxic effects. Primary endpoint was PFS as assessed in the intent-to-treat population, whereas OS was a secondary endpoint. Assessment of PFS according to the tumor EGF-R mutation status was also a preplanned exploratory objective.
Median duration of treatment was 148 [49-467] days with gefitinib and 73 [42-127] days with placebo. PFS was significantly longer with gefitinib than that with placebo [median PFS 4.8 (95% CI: 3.2-8.5) versus 2.6 months (1.6-2.8 months); hazard ratio 0.42; 95% CI: 0.33-0.55; P
Support for this proposal came from two other phase III studies. In the Japanese WJTOG0203 study (14), 595 patients were randomly assigned to receive either gefitinib after three cycles of platinum-based chemotherapy or six cycles of chemotherapy only. PFS was significantly prolonged in patients receiving a combination of chemotherapy and gefitinib (P
In addition, Gaafar and co-workers (15) reported a study of similar design and could also show a significantly improved PFS with gefitinib (P=0.0015) versus placebo whereas OS did not differ between the two treatment groups.
In this light, Lee and co-workers (5) included 23 eligible trials (13 front-line, 7 second-line, 3 maintenance; N=14,570) in a meta-analysis. EGF-R mutation status was known in 31% of patients. EGF-R-TKIs treatment prolonged PFS in EGF-Rmut (+) patients, and EGF-R mutation was predictive of PFS in all settings. EGF-R-TKIs therapy statistically significantly delays disease progression in EGF-Rmut (+) patients but has no demonstrable impact on OS. EGF-R mutation was shown to be a predictive biomarker of PFS benefit with EGF-R-TKIs treatment in all settings. These findings support EGF-R mutation assessment before initiation of (maintenance) treatment. EGF-R-TKIs such as gefitinib or erlotinib should therefore be considered as front-line therapy in EGF-Rmut (+) advanced NSCLC patients, a finding, that has also been confirmed by the results of the SATURN and the INFORM trial as well.
In contrast, Chen et al. (6) showed that NSCLC patients (meta-analysis, N=3,903) with clinical features such as female, never smoker, adenocarcinoma, Asian ethnicity and EGF-R mutation positive had more pronounced PFS benefit following maintenance therapy. OS benefit was observed in patients with clinical features such as female, non-smoker, smoker, adenocarcinoma, and previous stable to induction chemotherapy. They concluded that maintenance therapy with gefitinib produces a significant PFS and OS benefit for unselected patients with advanced NSCLC compared with placebo or observation suggesting that this treatment strategy may be of important clinical value.
Although pemetrexate and erlotinib have been approved as maintenance therapy for advanced NSCLC patients, the precise role for the treatment strategy of NSCLC in terms of a maintenance approach is far from being clear and additional studies are warranted to further clarify this option.
Moreover, despite these controversial findings, the question remains whether the benefit of maintenance therapy for NSCLC is best defined by PFS. Truely, PFS is the best predictor for improved OS (and is independent of subsequent treatment), but OS is acknowledged as the key clinical outcome in the treatment of advanced NSCLC. However, one has to keep in mind that OS will often be confounded by the subsequent treatments patients receive. Nevertheless, more future studies should therefore clearly focus on OS as a primary end point for any type of maintenance therapy in NSCLC patients.
The approval of pemetrexed and erlotinib by the FDA and the EMEA has certainly shifted the pendulum towards maintenance therapy. Yet despite these apparent advances, however, for most patients with NSCLC maintenance therapies with gefitinib or other drugs have not dramatically changed clinical outcome. The molecular complexity of lung cancer underlies these disappointments and stresses the need for optimizing treatment by seeking a more personalized approach to care. Therefore, clinical trials that investigate the activity of novel maintenance regimes, and incorporate patient selection based on clinical and molecular factors, are required.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13 Suppl 1:5-13. [PubMed]
- Shepherd FA. Second-line chemotherapy for non-small cell lung cancer. Expert Rev Anticancer Ther 2003;3:435-42. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer 2010;67:257-74. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [PubMed]
- Chen X, Liu Y, Røe OD, et al. Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: a systematic review. PLoS One 2013;8:e59314. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Xu JM, Han Y, Li YM, et al. Phase II trial of sequential gefitinib after minor response or partial response to chemotherapy in Chinese patients with advanced non-small-cell lung cancer. BMC Cancer 2006;6:288. [PubMed]
- Mencoboni M, Bergaglio M, Serra M, et al. Maintenance therapy with gefitinib after first-line chemotherapy in patients affected by advanced non-small cell lung cancer. Anticancer Res 2007;27:4425-9. [PubMed]
- Pallis AG, Christofillakis Ch, Tselepatiotis E, et al. Sequential administration of docetaxel followed by maintenance gefitinib, as salvage treatment in patients with advanced NSCLC: a multicenter phase II trial. Lung Cancer 2007;55:101-7. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Wang MZ, Zhong W, Zhang L, et al. Efficacy and safety of gefitinib in maintenance therapy for patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2008;30:221-4. [PubMed]
- Takeda K, Hida T, Sato T, et al. Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (WJTOG0203). J Clin Oncol 2010;28:753-60. [PubMed]
- Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer 2011;47:2331-40. [PubMed]
- Ahn MJ, Yang JC, Liang J, et al. Randomized phase II trial of first-line treatment with pemetrexed-cisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer 2012;77:346-52. [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [PubMed]


