Mechanisms of immune response regulation in lung cancer
Lung cancer is the main oncological problem worldwide. Lung cancer is the first cause of cancer death among patients with malignancy. There are more than 1,600,000 new cases yearly (1). The prognosis is very poor with less than 15% of the total survival (2). There was demonstrated a strong causal relationship of lung cancer with cigarette consumption (1).
There are two main histological types of lung cancer: non-small cell lung cancer (NSCLC) occurring in more than 85% of lung cancer cases and small cell lung cancer (SCLC). Both these types are totally different in biological character, clinical course and response to treatment. The basic curative method of treatment in NSCLC is tumor resection, while in SCLC-chemotherapy. The NSCLC is classified as a squamous cell type, adenocarcinoma and a large cell type (3). At the present time it is crucial to perform an appropriate histological classification and to select patients individually for treatment strategy with deep analysis of molecular tumor characteristic and evaluation of predictive factors (4-6). In the advanced stages of NSCLC chemotherapy and targeted therapy are being used with some effectiveness. Unfortunately chemotherapy is capable to destroy not only malignant cells but also normal tissue and immune cells, so to determine patient immune status before treatment seems to be very important. On the other hand some new agents used in chemotherapy sensitize immune system to immunotherapy by reducing the number of suppressor cells (7). The main causative agent for lung cancer development is tobacco smoke. However, in the last years the epidemiology of this malignancy changes with increasing incidence of lung cancer among non-smokers who never smoked (1). The last is considered to be a different disease: the adenocarcinoma type predominates, occurs most commonly in females and in patients at the older age (8). The total morbidity and mortality due to lung cancer are strongly correlated to the age. The senescence of immune system is a possible cause of this type of cancer. The following factors lead to lung cancer development in elderly: the impaired function of antigen presenting cells (APC), the increased concentration of IL-6, depletion of IL-2 with deficiency of effector T-cell population, Th2 polarization, the increased number of Th cells without costimulatory CD28 molecule (CD4/CD28null) (9,10). In all of the above the suppressor/regulatory properties prevail over the activation of immune system in immunosurveillance of cancer. The mechanism of immunosurveillance in lung cancer is complex and declined in the course of the disease and the immunological tolerance i.e., the unresponsiveness of the immune system to specific antigens develops (11) (Figure 1).
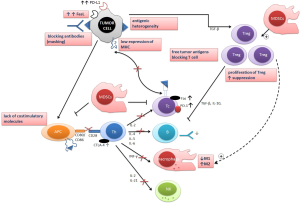
The key immune cells active in tumor killing are cytotoxic T Lymphocytes (CTLs). CTLs with other effectors [among others—natural killer (NK) cells] are recruited thanks to tumor antigen presence mainly by dendritic cells (DCs) and macrophages. However, the antigen presenting function in malignancy is impaired. The anticancer defensive function is balanced and then outweighed by the regulation of immune response (12). As well as cytotoxic response is complex, the suppression of host anticancer answer is realized by a composite network of cells and mediators modified by tumor microenvironment. This microenvironment is composed by tumor cells, fibroblasts, endothelial cells, macrophages, DCs, lymphocytes and the extracelluar matrix with many mediators: cytokines, chemokines, growth factors and enzymes (13). More other the tumor milieu is characterized by the altered conditions like hypoxia, which promotes angiogenesis and impact the epithelial-mesenchymal transition with increase metalloproteinase production (MMP-7, MMP-9). Tumor derived cytokines affect DCs thus the antigen presenting function is impaired. DCs in the course of cancer have immature phenotype and make T cells more tolerant to the autologous tumor cells (14,15).
The results of recent studies confirmed the important role of regulatory cells in the modification of immune response in malignancy. It was presented that regulatory T lymphocytes (Tregs) are capable to inhibit function of CD4+ and CD8+ lymphocytes, DCs and NK cells (16). Tregs play the important role in the immune surveillance and tolerance (16,17). Tregs are not numerous and not uniform lymphocyte subset [are present in peripheral blood (PB) in low proportion: 2-5% of lymphocytes] and are identified by the expression of the panel of the surface antigens. Tregs express: forkhead box P3 (Foxp3), CD25, glucocorticoid-induced TNF-receptor (GITR) (CD357), lymphocyte-activation gene 3 (LAG3), cytotoxic T lymphocyte antigen-4 (CTLA4) and downregulation of CD127 (IL-7R). However, Simonetta et al. observed high CD127 expression in the activated state (18). The specific inducing Tregs are triggered by the stimulation of tolerogenic DC, TGFβ and IL-10. The Tregs family is formed by: Tr1 (CD4+ cells induced by IL10), Th2, Th3 (acting by TGFβ), CD8+ cells, NKT (CD4–/CD8–) and “natural” T regulatory cells (Treg) (19). Natural Tregs are characterized by the expression of: CD4, CD25, Foxp3 and low CD127 and are derived from thymus, while induced Tregs (iTregs) are differentiated on periphery by cytokines and by contact with CD4+ cells (19). Foxp3 is a molecule which is necessary for proper Tregs function (20). Functionally the population of natural Tregs is derived from thymus having a strong suppressor function and expression of Foxp3, the second adaptive Tregs acquire Foxp3 expression after activation by antigenic exposure, known as a compression (20-22). The tumor milieu presents a good condition to conversion with specific antigenicity and high TGFβ concentration. DC plays a role in this process of conversion. The function of Tregs is widely explored. Recently Ju et al. described the population of CD13+/CD4/CD25high Tregs in the PB of lung cancer patients with strong inhibitory properties (23). At this point it is worth noting that the CTLA4 molecule which is costimulator for Tregs is presented as an important factor in tumor progression. CTLA4 is constitutively expressed on Tregs and plays a role in regulating T cell tolerance (16,24,25).
The number and the function of Tregs are significantly depressed in the autoimmune diseases (17,26-28) while in malignancy both are augmented (21,29,30). The increased expression of Foxp3 was found in the cancer cells of different kinds of tumors and in tumor infiltrating lymphocytes (TIL) (31-33). The presence of Tregs as well as the expression of Foxp3 in lung cancer was found as a negative prognostic factor (34). Targeting of regulatory T-cells and negative regulatory molecules have been used in the attempt to circumvent immune tolerance (12,30,35). A significantly higher proportion of Tregs population in tumor and lymph nodes than in PB of lung cancer patients identified by flow cytometry was recently reported by Shigematsu et al. (36). In the other studies the Foxp3 expressing cells were detected by immunohistochemistry and their presence in tumor as well as in TIL was a negative prognostic factor (34,37-39). In the study of Tao et al. the Foxp3 expression was present in about one third of tumor cells. The prognostic importance was proposed when accompanied by Tregs infiltration: Foxp3 on tumor cells seemed to be a favorable factor for survival. The authors proposed Foxp3/Tregs index as a useful factor for prognosis using the same method for Foxp3 detection in cancer cells as well in lymphocytes (39). Of the other hand Liu et al. concluded that high Foxp3/CD8 ratio in biopsy specimens of patients with advance lung cancer is a predictor to response to chemotherapy (37). In the study of Fu et al. the Foxp3 was highly expressed in NSCLC cells and correlated with Toll like receptor 4 (TLR4) expression. TLR4 is present in lymphoid and tumour cells and promotes the immune tolerance in cancer by release of many active inflammatory mediators (40). Recently, Ju et al. defined highly suppressive cells with the phenotype: CD13+/CD4+/CD25high Tregs which proportion in the PB correlated with the pathological stage of NSCLC. These cells expressed high levels of proteins known to have a strong immunosuppressive function: CTLA-4, mTGFβ1 and B7-H1 (PD-L1) (CD274) (23). The PD-L1 is the ligand to programmed death receptor-1 (PD-1), which is present on cytotoxic cells. The PD-1-PD-L1 interaction has a strong immunosuppressive effect and it was documented the blockade of PD-1/PD-L pathway using a fully humanized anti-PD-1 antagonistic mAb increases the numbers and functions of tumor-specific T cells. Tregs are differentiated in the tumor environment by cytokines released by tumor cells and lymphoid cells: mainly by IL-10 and TGFβ. It was also found that Tregs are recruited from thymus by thymic stromal lymphopoietin (TSLP) by DC activation. The expression of TSLP was shown to be increased in lung cancer tissue and correlated with Tregs proportion (22). CD8+ Tregs are unknown in normal conditions however, in tumor environment the population of CD8/CD25+ and CD8/CD28– was described (7) with a higher proportion of the last population in the PB of lung cancer patients (41).
The other mechanism of the impaired anticancer defense and cancer cells hiding is the alterations of costimulatory molecules in cancer cells and in APC (11). T cells are the main cytotoxic population and recognize target cells by the action of APC (14). B7 molecule in the APC and their receptor CD28 in lymphocyte are necessary to activate their cytotoxic effect. B7 molecule is capable to present the suppressive signal by action with CTLA4, which inhibit the TCR signal on T cells (25). The augmentation of Tregs is accompanied by a higher expression of CTLA4. CTLA4 is 30% homological with CD28—costimulatory molecule with about 40× stronger affinity. By blocking CD28 CTLA4 leads to the depletion of a cell cycle, the decreased release of IL-2 and increased TGFβ production. CTLA4 is present only in TCR activated CD4 and in smaller number in CD8 cells. CTLA4 is needed for the regulation of activation: the CTLA4 knocked out mice develop sever lymphoproliferative disease with a massive polyclonal T-cell infiltration in organs (42). A gene encoding CTLA4 is a target for Foxp3 so CTLA4 is constitutively expressed on Tregs. This molecule appears on the surface of the cells after activation and is rapidly internalized into the cellular storage. It was found in NSCLC that the CTLA4 proportion was higher intracellular than on the surface of circulating CD4+ cells (43,44). However, the precise mechanism by which CTLA4 enhance immunosuppressive Tregs function is unknown. The antibody blocking CTLA4 is under investigation in lung cancer immunotherapy.
Cancer cells release many cytokines capable to show a suppressive immune effect in cancer microenvironment. The best recognized is TGFβ. The increased concentration of TGFβ in cancer tissue, bronchoalveolar lavage fluid from lung affected by cancer and the culture of cancer cells were reported (45-47). The following functions of TGFβ are strictly connected with the tumor progression: the inhibition of NK and CD8 cells function, the differentiation of Th to Th2 profile and the preservation of Tregs differentiation (48). On the other hand tumor cells are insensitive to inhibitory properties of TGFβ because of the loss of TGFβ receptors (49). Some interleukins present similar effect, among others IL-10 and IL-2, which induces CTLA4. IL-10 is present in high concentration when released by tumor cells as well as by immune cells. High concentration of IL-1, IL-6, IL21 and IL-23 in cancer milieu leads to the next part of the regulatory network in anticancer response i.e., Th17 cell differentiation (50). Th17 is a lineage of Th cells which is additional to the type Th1 and Th2. Th17 cells are defined by production of IL-17A. It was shown that the mouse Th17 has a common pathway with Treg Foxp3+. Both cell populations are produced after TGFβ stimulation but the differentiation to Th17 is mediated by IL-6. It is presumed that IL-6 inhibits Treg development with stimulation of Th17 TGFβ depended (51). In humans the interleukins: IL-1β and IL-23 are further required to the Th17 proliferation. The polarization Th17 is initiated by IL23 via IL23 R and Stat3 pathway to Th secreting IL17A. The second and stronger way is generated by TGFβ and IL6 via Smad family proteins (52). TGF at a low concentration contributes to the Th17 differentiation, while in high concentration to Foxp3 iTregs differentiation. These relations reflect a permanent balance between cytokines and Th populations and the Th17 plasticity. Th17 lymphocytes are active in antimicrobial defense, but their proliferative and cytotoxic effects are low. The role of Th17 cells in autoimmunity was documented (53). There are no direct data on the anticancer effect of Th17. Till now same results indicate that the effect of Th17 is complex as the IL-17 action in cancer milieu is pleiotropic: suppressive and stimulating (54). The stimulating effect is related to proangiogenic role of IL-17 (55). IL17A was present in high levels in a cancer tissue, PB and may stimulate angiogenesis by VEGF, recruit neutrophils and act as a tumor promoter (49,56). The immunosuppressive action of Th17 was also described (54). Recently, Zheng et al. described the IL-23R gene polymorphism connected with Tregs function and modification the susceptibility to lung cancer (57).
The main role of the cells originated from monocytes is antigen presentation. However recent data documented that this cell lineage plays a considerable regulatory function. In the solid tumors the population of tumor associated macrophages (TAM) was widely investigated. TAM are monocytes and local macrophages recruited to solid tumors playing several roles in tumor progression, rather promotion than suppression. Their infiltration, which may be as great as 50% of tumor mass, results from the so-called “cancer education” (58-60). In general the function of TAM is impaired. Macrophages were previously considered as a uniform cell population, recently these cells are divided into different phenotypes: M1 and M2 and macrophages with regulatory properties (58,61). M1 and M2 are activated by different way and this polarization depends on the character of activation rather than the phenotype or the kind of released mediators. M1 are activated by LPS and INF while M2 by IL-4, IL-10, IL-13 and TGFβ (61). M1 macrophages are the effector cells, they play the immunostimulating role with secreting amounts of cytokines (IL-12 among others) and have phagocytic properties. The presence of M1 in TAM was found to be a favorable for survival in NSCLC (62). M2 macrophages with their suppressive function form about 70% of TAM population in NSCLC and promote angiogenesis, wound healing, release IL-10 (58,63). Recently Liu et al. described the relationship of M2 polarization in NSCLC environment with IL-17 and prostaglandin E2 (63). This different polarization of macrophages may be also detected by the immunocytochemistry with the following antibodies anti: CD206, CD163 (M2) and CD40 (M1). For regulatory macrophages (RMs) no defined surface antigenity is known, so the identification is based on the release of TGFβ and IL-10 (61). RMs present similar features to M2 producing high levels of IL-10 and being induced by IL4/IL13 network. These regulatory cells also express high levels of arginase. The Foxp3+ macrophage population inhibiting immune response was described in mice, however the strictly defined population of such macrophages in humans is unknown. The close functional relationship of Tregs with M2 macrophages was described in mice: CD4+/CD25+ cells are capable to enhance the expression of CD23, CD47, CD206, Toll Like Receptor 2 (TLR2) and TLR4 on macrophages and to potentiate the production of IL-10, TGFβ and arginase by macrophages (64). The investigation of TAM is possible in the samples from resected tumors while only about 30% cases of NSCLC are resectable; SCLC is nonresectable per se, so in the most of lung cancer cases the tumor microenvironment is not available for investigation. Nonetheless at the disposal of researchers there is BAL, the method of investigation of large part of the lung which provides the examination of cellular as well as humoral immune response (65,66). Alveolar macrophages present about 80% of BAL cells. Our previous investigation on the expression of ICAM-1 on BAL macrophages after stimulation by INFγ in lung cancer patients documented the usefulness of this method (67).
Myeloid derived suppressor cells (MDSCs) present a subpopulation of bone marrow derived hematopoietic cells, precursors of macrophages, granulocytes and DCs. The expansion of MDSCs in circulation was observed in sepsis, autoimmune disease as well as in malignancy (68,69). In NSCLC the lineage of MDSCs was defined by: CD11+CD14-CD15+CD33+ markers. The mediators secreting by cancer cells: GM-CSF, IL-6, IL1 support the survival of MDSCs in microenvironment. MDSCs inhibit activation of T cells, DC differentiation and promote Tregs. The mechanism of their action controlling T cell response involve the activity of arginase, nitric oxid (NO) synthase, the production of radical oxygen species (ROS) and the cysteine consumption (arginine, cysteine and NO are necessary for a proper T cell activation and memory type differentiation). MDSCs are capable to produce IL-10, MMP9 and TGFβ and favor angiogenesis, vasculogenesis and metastases (70). The COX2 overexpression in lung cancer and the process of epithelial-mesenchymal transition (EMT) seem to play an important role in the inhibition of antitumor response by MDSCs (7,71). EMT is necessary for tumor cells spread and it acts with MDSCs. The participation of transcription factors in this process was described. The recognition of MDSCs function leads to the development of therapy targeting these cells (12).
Conclusions
Lung cancer belongs to solid tumors of low antigenicity, heterogeneous and has a relatively low availability to research resulting from the low resection rate: about 70% of NSCLC cases are in the advanced stages at recognition and can’t be qualified for curative treatment. The review of the literature shows that the studies on lung cancer immunity are being performed on resected tumors, samples of bronchial biopsies, PB, experimental studies or cancer cell lines. A very useful method but, so to say “wasteful”, is the analysis of cells and mediators in the BAL. BAL may be performed in the advanced stages of lung cancer cases and in patients with SCLC. Our previous studies confirmed usefulness of this relatively low invasive method in the evaluation of immune status in the course of lung cancer (46,65,66,72,73). Taking into account the new promising results of immunotherapy and the tendency to personalized therapy to recognize the immune status before treatment is very important. It was mentioned that “the established tumors often also have the established regulatory cell population” and it is particularly essential to assess the population of Tregs (CD4/CD25high/FoxP3/CTLA4), M2, MDSCs and some cytokines: TGFβ, IL-10, IL-17A (30,74). The description of the immune status before treatment seems to be a new challenge for pathologists apart from the recognition of the histologic type of lung cancer and identification of molecular alterations (EFGR, ALK, KRAS).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-29S.
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [PubMed]
- Dail DH, Hammar SP, Colby TV. Pulmonary Pathology Tumors. Springer-Verlag, 1995.
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [PubMed]
- Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer 2010;67:257-74. [PubMed]
- Thunnissen E, Kerr KM, Herth FJ, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung Cancer 2012;76:1-18. [PubMed]
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther 2012;12:923-37. [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [PubMed]
- Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244-54. [PubMed]
- Fulop T, Larbi A, Witkowski JM, et al. Aging, frailty and age-related diseases. Biogerontology 2010;11:547-63. [PubMed]
- Bright RK. Immunology of lung cancer. In: Pass HI, Mitchel IB, Johnson DH, et al. eds. Lung Cancer. Lippincott W&W, 2000:304-18.
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381-8. [PubMed]
- Witz IP. Tumor-microenvironment interactions: the selectin-selectin ligand axis in tumor-endothelium cross talk. Cancer Treat Res 2006;130:125-40. [PubMed]
- Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530-51. [PubMed]
- Nicod LP, Cochand L, Dreher D. Antigen presentation in the lung: dendritic cells and macrophages. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:246-55. [PubMed]
- Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol 2004;16:89-98. [PubMed]
- Nielsen J, Holm TL, Claesson MH. CD4+CD25+ regulatory T cells: II. Origin, disease models and clinical aspects. APMIS 2004;112:642-50. [PubMed]
- Simonetta F, Chiali A, Cordier C, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 2010;40:2528-38. [PubMed]
- Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol 2006;16:137-49. [PubMed]
- Sainz-Perez A, Lim A, Lemercier B, et al. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res 2012;72:3557-69. [PubMed]
- Kryczek I, Liu R, Wang G, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 2009;69:3995-4000. [PubMed]
- Li H, Zhao H, Yu J, et al. Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol Immunother 2011;60:1587-96. [PubMed]
- Ju S, Qiu H, Zhou X, et al. CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function and increase with tumor stage in non-small cell lung cancer patients. Cell Cycle 2009;8:2578-85. [PubMed]
- Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565-94. [PubMed]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. [PubMed]
- D’Ambrosio D. Regulatory T cells: how do they find their space in the immunological arena? Semin Cancer Biol 2006;16:91-7. [PubMed]
- Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 2004;200:277-85. [PubMed]
- Domagała-Kulawik J, Hoser G, Dąbrowska M, et al. CD4+/CD25+ cells in systemic inflammation in COPD. Scand J Immunol 2011;73:59-65. [PubMed]
- Karanikas V, Speletas M, Zamanakou M, et al. Foxp3 expression in human cancer cells. J Transl Med 2008;6:19. [PubMed]
- Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012;62:309-35. [PubMed]
- Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9:606-12. [PubMed]
- Douglass S, Ali S, Meeson AP, et al. The role of FOXP3 in the development and metastatic spread of breast cancer. Cancer Metastasis Rev 2012;31:843-54. [PubMed]
- Xue L, Lu HQ, He J, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus 2010;23:340-6. [PubMed]
- Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006;107:2866-72. [PubMed]
- Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer 2011;73:11-7. [PubMed]
- Shigematsu Y, Hanagiri T, Shiota H, et al. Immunosuppressive effect of regulatory T lymphocytes in lung cancer, with special reference to their effects on the induction of autologous tumor-specific cytotoxic T lymphocytes. Oncol Lett 2012;4:625-630. [PubMed]
- Liu H, Zhang T, Ye J, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother 2012;61:1849-56. [PubMed]
- Schneider T, Kimpfler S, Warth A, et al. Foxp3(+) regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol 2011;6:432-8. [PubMed]
- Tao H, Mimura Y, Aoe K, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 2012;75:95-101. [PubMed]
- Fu HY, Li C, Yang W, et al. FOXP3 and TLR4 protein expression are correlated in non-small cell lung cancer: implications for tumor progression and escape. Acta Histochem 2013;115:151-7. [PubMed]
- Karagöz B, Bilgi O, Gümüs M, et al. CD8+CD28- cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol 2010;27:29-33. [PubMed]
- Mocellin S, Nitti D. CTLA-4 blockade and the renaissance of cancer immunotherapy. Biochim Biophys Acta 2013;1836:187-96.
- Erfani N, Mehrabadi SM, Ghayumi MA, et al. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer 2012;77:306-11. [PubMed]
- Erfani N, Khademi B, Haghshenas MR, et al. Intracellular CTLA4 and regulatory T cells in patients with laryngeal squamous cell carcinoma. Immunol Invest 2013;42:81-90. [PubMed]
- Fischer JR, Darjes H, Lahm H, et al. Constitutive secretion of bioactive transforming growth factor beta 1 by small cell lung cancer cell lines. Eur J Cancer 1994;30A:2125-9. [PubMed]
- Domagała-Kulawik J, Hoser G, Safianowska A, et al. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp (Warsz) 2006;54:143-7. [PubMed]
- Bennett WP, el-Deiry WS, Rush WL, et al. p21waf1/cip1 and transforming growth factor beta 1 protein expression correlate with survival in non-small cell lung cancer. Clin Cancer Res 1998;4:1499-506. [PubMed]
- Través PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm 2012;2012:568783.
- Andreev K, Graser A, Maier A, et al. Therapeutical measures to control airway tolerance in asthma and lung cancer. Front Immunol 2012;3:216. [PubMed]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133-41. [PubMed]
- Romagnani S. Human Th17 cells. Arthritis Res Ther 2008;10:206. [PubMed]
- Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood 2013;121:2402-14. [PubMed]
- Martinez GJ, Nurieva RI, Yang XO, et al. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci 2008;1143:188-211. [PubMed]
- Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011;186:4388-95. [PubMed]
- Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 2005;175:6177-89. [PubMed]
- Li Y, Cao ZY, Sun B, et al. Effects of IL-17A on the occurrence of lung adenocarcinoma. Cancer Biol Ther 2011;12:610-6. [PubMed]
- Zheng J, Jiang L, Zhang L, et al. Functional genetic variations in the IL-23 receptor gene are associated with risk of breast, lung and nasopharyngeal cancer in Chinese populations. Carcinogenesis 2012;33:2409-16. [PubMed]
- Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549-55. [PubMed]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-69. [PubMed]
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004;104:2224-34. [PubMed]
- Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal 2011;11:2391-402. [PubMed]
- Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010;10:112. [PubMed]
- Liu L, Ge D, Ma L, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol 2012;7:1091-100. [PubMed]
- Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol 2011;89:130-42. [PubMed]
- Domagała-Kulawik J, Hoser G, Droszcz P, et al. T-cell subtypes in bronchoalveolar lavage fluid and in peripheral blood from patients with primary lung cancer. Diagn Cytopathol 2001;25:208-13. [PubMed]
- Domagała-Kulawik J, Guzman J, Costabel U. Immune cells in bronchoalveolar lavage in peripheral lung cancer--analysis of 140 cases. Respiration 2003;70:43-8. [PubMed]
- Dabrowska M, Grubek-Jaworska H, Hoser G, et al. Effect of IFN-gamma stimulation on expression of intercellular adhesion molecule-1 (ICAM-1) on alveolar macrophages in patients with non-small cell lung cancer. J Interferon Cytokine Res 2006;26:190-5. [PubMed]
- Srivastava MK, Dubinett S, Sharma S. Targeting MDSCs enhance therapeutic vaccination responses against lung cancer. Oncoimmunology 2012;1:1650-1651. [PubMed]
- Srivastava MK, Zhu L, Harris-White M, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012;7:e40677. [PubMed]
- Srivastava MK, Andersson Å, Zhu L, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291-304. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [PubMed]
- Domagała-Kulawik J, Droszcz P, Kraszewska I, et al. Expression of Fas antigen in the cells from bronchoalveolar lavage fluid (BALF). Folia Histochem Cytobiol 2000;38:185-8. [PubMed]
- Hoser G, Domagała-Kulawik J, Droszcz P, et al. Lymphocyte subsets differences in smokers and nonsmokers with primary lung cancer: a flow cytometry analysis of bronchoalveolar lavage fluid cells. Med Sci Monit 2003;9:BR310-5. [PubMed]
- Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012;13:e301-10. [PubMed]


