Pragmatic trial of a multidisciplinary lung cancer care model in a community healthcare setting: study design, implementation evaluation, and baseline clinical results
Introduction
With an estimated incidence of 224,500 new cases and 158,000 deaths, lung cancer accounted for 14% of all cancer cases and 25% of all cancer deaths in the US in 2016. It kills almost as many Americans each year as the second (colorectal), third (pancreas), fourth (breast), and fifth (prostate) most lethal cancers combined. While aggregate 5-year survival rates have improved significantly for breast (90%), colorectal (60%) and prostate (95%) cancers, the overall 5-year survival rate for lung cancer has only increased from 12% in 1975 to 18% in 2016 (1).
Lung cancer presents unique challenges in oncologic care delivery due to the cumulative age and tobacco-related comorbidities of many patients. The median age at diagnosis of lung cancer in the US is 72 years, >30% of patients have co-morbid illness, including 20% who are survivors of a previous cancer (2,3). The chest cavity and mediastinum, the anatomic location of potentially curable lung cancer, are relatively inaccessible. Lung cancer requires the active engagement of highly-trained, highly-skilled practitioners, using relatively high-cost, high-risk equipment and procedures to perform routine tasks required for diagnosis, staging, treatment and surveillance. Different specialists are often needed at each phase of care, which fragments care delivery (4,5).
The current standard of care for patients with lung cancer involves multiple providers, potentially from multiple institutions or physician groups, and requires that patients move through a sequence of serial referrals. For example, diagnosis alone involves a minimum of 4 specialist referrals: the practitioner who ordered the initial radiologic study (often a primary care, emergency room, or hospitalist practitioner), a diagnostic radiologist, a diagnostic tissue procurer (usually an interventional radiologist, pulmonologist, or surgeon), and a pathologist. Staging and treatment modalities are increasingly optimally combined, such as use of radiologic and invasive staging methods, and combinations of surgery, chemotherapy, radiation therapy, and palliative care for treatment (6,7). These require engagement of additional specialists.
In the prevalent serial model of care-delivery, the level of contact and coordination between providers varies greatly, but is often minimal. The level of mutual self-identification as members of a team providing care is usually low. Therefore, active interdisciplinary engagement is a frequently recommended strategy for quality improvement (6-9). The multidisciplinary care model involves multiple providers working consciously together as a team to provide care. However, what constitutes multidisciplinary cancer care has not been well defined, and its implementation has not been thoroughly studied (4,5).
Consequently, there is a wide gap between the consensus expert recommendation for multidisciplinary care, which makes intuitive sense, and real-world examples of well-executed, functional multidisciplinary oncology care programs. This poor penetration of multidisciplinary care is partly caused by a paucity of evidence to support the benefit, and near-absence of implementation know-how (4,5). There are few rigorous studies and little high-quality data to justify the resource investment and disruption of existing structures and relationships required to implement the multidisciplinary care model. Meaningful multi-perspectival endpoints need to be defined and evaluated to measure the ‘real-world’ impact of implementing multidisciplinary care. Given its complexity, lung cancer offers fertile ground for testing multidisciplinary models of care in oncology practice.
We sought to evaluate the implementation of a rigorously benchmarked multidisciplinary lung cancer care model involving early, concurrent, and prospective engagement of key physician specialists in a co-located weekly clinic. Our goals were to develop meaningful, measureable benchmarks, and to define process endpoints related to quality of care, patient and caregiver reported outcomes, and survival. Using a prospective cohort study design, we compared processes and outcomes within multidisciplinary and usual serial care delivery environments within the same healthcare system. In this report we describe the study design, evaluate implementation, and compare baseline clinical characteristics.
Methods
We used a mixed-methods approach to conduct a patient-centered, multi-phase combined implementation and effectiveness study of the multidisciplinary model of lung cancer care within a large not-for-profit Comprehensive Community Cancer Program in a high lung cancer incidence and mortality region of the US. The study was conducted in three phases. In phase 1, we used focus groups to assess direct stakeholder input from patients, their caregivers, doctors, nurses, hospital administrators, and senior executives of national health insurance companies. The objective of phase 1 was to solicit stakeholder perspectives on optimal lung cancer care delivery, the relative advantages and disadvantages of the multidisciplinary and serial models of care delivery, obstacles to implementation of multidisciplinary care, and benchmarks for the evaluation of quality of care delivery (10).
In phase 2, we evaluated the initial implementation of a carefully benchmarked multidisciplinary clinic based on stakeholder-relevant endpoints. Key measures included: the rate of concordance between consensus recommendations from the multidisciplinary panel and actual delivered care, rate of successful administration of survey instruments, verifiable rates of communication of specific vital clinical information between clinicians and patients and their caregivers, and among all clinicians responsible for the patient’s care. After a lead-in phase with consistent attainment of pre-set targets on each of the 3 process measures, the project transitioned to phase 3, a prospective comparative effectiveness trial to determine the impact of multidisciplinary care on stakeholder-relevant clinical outcomes compared with patients receiving serial care. The comparative effectiveness endpoints include: patient and caregiver reported outcomes obtained through surveys administered at baseline, 3 and 6 months after enrollment; the thoroughness and accuracy of staging indicated by rates of utilization of recommended staging tests and the rates of stage evolution from baseline to just before onset of treatment; the rates of deployment of guideline-recommended treatment modalities based on stage; the timeliness of care; and survival.
This study was approved by the Baptist Memorial Healthcare Corporation Institutional Review Board (IRB) and the University of Memphis IRB (ID: 3385). All participants in the qualitative studies, and patients and caregivers in the comparative effectiveness study provided written informed consent.
Phase 1: eliciting stakeholder perspectives
Pre-planning (initial concept development) included multiple stakeholders, including a patient and his caregiver, a medical oncologist, thoracic surgeon, nurse navigator, hospital administrators, and a corporate attorney. After the initial preliminary planning phase, additional stakeholders were identified to form a Steering Committee, including additional patients, caregivers, epidemiologists, a clinical psychologist, an implementation scientist, a medical anthropologist, a representative from the American Cancer Society, a palliative care nurse, and a representative of a local Federally Qualified Health Center.
Qualitative evaluation of key stakeholders
We identified the key stakeholders as lung cancer patients and their caregivers (the consumers of care), physicians and nurses (the providers of care), healthcare administrators (providers of the environment of care-delivery), and health insurance company executives (the payers). We broadly categorized physicians into 2 groups: ‘first responders’ (primary care, emergency care and hospital care practitioners) and ‘involved specialists’ (thoracic surgeons, pulmonologists, medical and radiation oncologists, palliative care specialists). The first-responders typically request the diagnostic studies from which the presence of lung cancer is initially detected but do not have the expertise to provide necessary lung cancer care and therefore have a referral mandate. For them, the multidisciplinary program potentially had a readily identifiable service benefit. The involved specialists have specific skillsets required for the diagnosis, staging or treatment of lung cancer and potentially deem a multidisciplinary program as more of a threat to their practice autonomy.
We conducted 21 focus groups of key stakeholders. Participants included 22 patients, 24 caregivers, 9 nurses, 8 hospital administrators, 4 executives of health insurance companies, and 39 physicians. The physicians included primary care, hospitalists, pulmonologists, thoracic surgeons, medical oncologists, and radiation oncologists. Stakeholder input was compiled using qualitative methods and used to inform the make-up of the multidisciplinary clinic and the benchmarks for the comparative effectiveness study (10,11). Stakeholder feedback from the pre-planning suggested that broad access to care, patient satisfaction, timely care, and clear communication between patients and physicians were high priorities (10,11).
Phase 2: implementing the multidisciplinary clinic model of care
We started a prospective multidisciplinary Thoracic Case Conference in November 2011, and began a pilot implementation of the co-located multidisciplinary thoracic clinic in August 2012. After 2 years of strategic planning and development, including establishment of data collection processes, feedback and benchmarking, we established the Baptist Cancer Center Multidisciplinary Thoracic Oncology Clinic in Memphis, TN, with specific infrastructure to study the implementation of this model of care delivery.
The structure and activities of the program were implemented with direct stakeholder input. Performance benchmarks were defined by interactive group consensus and codified in written Standard Operating Procedures, to provide an objective measure of clinic function. The benchmarks were to: (I) maintain a concordance rate >85% between consensus management recommendations and actually delivered care; (II) obtain satisfaction score responses from ≥60% of patients, caregivers, and providers; (III) maintain patient and caregiver satisfaction scores of 4 (‘satisfied’) or 5 (‘highly satisfied’) on a Likert scale in >80% of patients in surveys administered after initial patient evaluation in the clinic; (IV) maintain provider satisfaction scores of 4 (‘satisfied’) or 5 (‘highly satisfied’) on a Likert scale in >70% of providers in surveys administered after each provider’s active interaction with the clinical program, either by direct participation (for participating providers) or after referring five patients into the program (for non-participating providers); (V) maintain communication between providers, measured by the timeliness of official verifiable communication of management decisions with providers in and outside the program and verifiable communication of management plans with patients and their caregivers. The minimal content of the communication was pre-specified to include histologic diagnosis, stage, and management recommendations, including details of what, who, where, and when. The goal was to have verifiable communication with all patients and their identified caregivers, and providers connected with each patient, within 48 hours of recommendations being made in >80% of patient clinic visits.
Phase 3: prospective comparative effectiveness study
Comparative groups
In this study, we compared patients categorized into two primary groups: multidisciplinary care vs. serial care. In the primary analysis, the multidisciplinary care arm consisted of patients (and their caregivers) who received care in the co-located clinic involving active participation of a thoracic surgeon, medical oncologist, radiologist, pulmonologist, and radiation oncologist, with active coordination of care by a dedicated Nurse Navigator before onset of treatment. All patients seen in the co-located clinic were also discussed in the Multidisciplinary Thoracic Oncology Conference, which involved a larger group of clinicians from each of the above-specified specialties, as well as a pathologist, and palliative care specialist. In the primary analysis, patients on the serial care arm received care by a variety of physicians, but were not seen in the co-located multidisciplinary clinic prior to onset of treatment.
A subgroup of these ‘serial care’ patients was discussed in the Multidisciplinary Thoracic Oncology Conference, and presumably thereby received a modicum of multidisciplinary input, but did not have any direct interaction with the Nurse Navigator nor were they seen in the co-located clinic. This ‘conference-only’ subset of ‘serial care’ patients provided a pragmatic opportunity to compare outcomes between the co-located clinic model and the ‘Tumor Board’ model of multidisciplinary care.
In our primary analyses, we combined the ‘conference only’ and ‘true’ serial care patients (serial care patients who were never discussed in the conference before onset of treatment) as the ‘serial care’ group for comparison to the multidisciplinary care group. In secondary analyses, we will evaluate the impact of the multidisciplinary conference by separating out the two serial care subsets for comparison to the multidisciplinary clinic cohort. Therefore, primary analyses compare two groups (multidisciplinary care vs. serial care), and secondary analyses will compare three groups (multidisciplinary care vs. conference only vs. true serial care). Future evaluation will also test the impact of combining the ‘conference only’ subset with the co-located multidisciplinary care patients for comparison to the ‘true’ serial care patients.
Recruitment and screening
Provider referrals
To increase clinician awareness across the healthcare system and prompt patient referrals to the multidisciplinary clinic, a marketing campaign was targeted at primary care providers, emergency room physicians, hospitalists, radiologists, thoracic surgeons, medical and radiation oncologists, pulmonologists and nurses. Likely point-of-entry physicians were informed of the clinic with specific marketing. The marketing plan included having the thoracic team members attend physician meetings, creating a specific announcement sent to all physicians to introduce the program, clinic site visits, along with hosting luncheons for physicians to be introduced and updated on the multidisciplinary program. Expanding access to a wider range of physicians in the new clinic environment was a task that developed over the course of several months.
Eligibility criteria
All patients with lung cancer, irrespective of stage or histology, confirmed within 8 weeks of eligibility evaluation, with an Eastern Cooperative Oncology Group (ECOG) Performance Status from 0 (asymptomatic) to 2 (symptomatic but out of bed for >50% of the day), no previous history of lung cancer, and no previous history of other invasive cancer within the past 5 years (excepting non-melanoma skin cancer), were eligible for the prospective comparative effectiveness study. Patients were eligible for enrollment in the multidisciplinary arm if they were seen within the co-located multidisciplinary clinic before onset of definitive treatment; patients on the serial care arm were eligible if within 4 weeks of treatment onset.
Data collection
Detailed prospective data collection was conducted by full-time data managers to ensure comprehensive information was available for each patient. Standard demographic and clinical information were collected on each patient, including age, race, sex, health insurance, histologic diagnosis, dates and location of clinical events. Data were obtained from all relevant providers from the initial detection of the lesion to the definitive treatment(s) (surgery, radiation therapy, chemotherapy, hospice care, no treatment). All data were independently audited for accuracy by a data manager different from the initial abstractor.
Comparative study processes
The multidisciplinary conference was held weekly from 7:00 am to 8:30 am on Wednesdays. Patients and caregivers were not present during the conference. Consensus recommendations were generated, prospectively recorded, and communicated to responsible physicians with requested acknowledgement of receipt, but no active engagement of the Nurse Navigator. The multidisciplinary clinic also met weekly from 10:00 am to 5:00 pm on Wednesdays. Patients were concurrently evaluated by potentially treating clinicians with direct patient and caregiver input solicited before final recommendations were made. Recommended care was communicated directly to patients in writing and the execution of care was coordinated by the Nurse Navigator. All patients seen in the co-located clinic were also presented in the multidisciplinary conference for broader consensus discussion, often with additional information generated from tests performed after the initial clinic visit.
Eligible and consenting patients were recruited into the multidisciplinary arm after initial visit to the multidisciplinary clinic. Care provided within the multidisciplinary clinic was focused solely on treatment planning (diagnosis, staging and triage into treatment pathways). Over the same period, with the permission of their lung cancer specialist provider (surgeons, medical oncologists, radiation oncologists), eligible and consenting patients were recruited into the serial care arm from clinics where they routinely sought care for lung cancer. All patients, irrespective of enrollment arm, received their treatment (surgery, radiation, chemotherapy, palliative care) within the same group of locations.
Data, measurements and instruments
We abstracted clinical data prospectively from the electronic health records, obtained missing and additional follow-up information by direct contact between clinical research coordinators and patients, caregivers, and patients’ physicians. Clinical data included all lung-cancer related events from initial diagnosis through the last modality of treatment of the patient’s lung cancer, including history and physical examination findings, radiologic and pathologic test results, physician consultations (within and outside the healthcare system), the multidisciplinary conference and clinic recommendations (as applicable), a summary of pre-treatment disease characteristics, details regarding treatment received (surgery, chemotherapy, radiation therapy and palliative care), post-surgery findings (such as histology and pathologic stage), and post-treatment outcomes. All clinical data for phase 3 patients were audited for accuracy by a data manager other than the initial abstractor.
Data regarding patient-related decisions, events, and communication that happened specifically within the lung cancer conference or multidisciplinary clinic were recorded by the multidisciplinary program’s two clinical research coordinators. We administered surveys to patients and their self-identified caregivers at baseline, 3 and 6 months. Respondents chose either paper or iPad administration and were given the option of responding in person, over the phone, or by mail. For patients and caregivers, we measured psychological distress with the Hospital Anxiety and Depression Scale (HADS) (12), satisfaction with care with the Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey (13), patients’ health-related quality of life (HRQOL) with the Functional Assessment of Cancer Therapy—Lung Cancer (FACT-L) (14), and caregivers’ HRQOL with the 36-Item Short Form Survey (SF-36) (15). Here we only report patients’ baseline HRQOL.
Clinical effectiveness measurements
The effectiveness of the multidisciplinary clinic services was measured by 6 key elements: timeliness of care, thoroughness of staging, stage-appropriate treatment, patient reported outcomes, caregiver reported outcomes, and survival. Timeliness of care was measured in days from initial detection of the radiologic lesion to onset of key steps in treatment including: diagnostic biopsy, non-invasive staging test, invasive staging biopsy, physiologic clearance for treatment, and initiation of definitive treatment. Thoroughness of staging was multifaceted, including the rates of histologic biopsy (or radiologic corroboration, for difficult to biopsy targets such as brain or bone lesions) of stage-defining lesions (the stage confirmation rate) and the rates of multimodal staging—using the combination of PET/CT scan or CT scan and invasive staging biopsy (bimodal staging), or CT scan, PET/CT scan and invasive staging biopsy (trimodal staging) (16). Stage-appropriate treatment was evaluated by examining stage-stratified definitive treatment rates as recommended by the National Comprehensive Cancer Network (NCCN) guidelines (6,7).
Survival
Overall survival and progression free survival were measured from the date of histologic diagnosis until last follow-up, based on data validated by 3 sources: state registry databases, clinical record reviews, and direct contact of consented patients or their caregivers.
Power calculations and statistical analysis
Overall survival was the primary endpoint and the study was powered at 80%, with alpha of 0.05, to detect a hazard ratio of 1.25 between patients receiving multidisciplinary care and serial care. We targeted enrollment of 150 patients on the multidisciplinary arm and 300 patients on the serial care arm, matched by clinical stage (based on the initial diagnostic CT scan), performance status, insurance status, race, and age range. After initiation of the study, it was determined that the individual 1:2 matching strategy was not attaining high level matches within the allotted parallel time frame in a substantial number of subjects. Therefore, a frequency matching strategy was used based on the matching variables (age was controlled for as a model covariate for better numerical efficiency). All comparative analyses were conducted using matching variables as strata, per original design, with the strata now allowing multiple cases and controls per match group (frequency matching). Specifically, analyses were designed based on conditional logistic regression for each binary endpoint and stratified cox proportional hazards models for survival endpoints (not reported here). For each outcome, additional statistical models were evaluated controlling for matching variables as covariates in unconditional analyses. All analyses were conducted in SAS Version 9.4 (Cary, NC, USA) with a type-I error rate of 0.05.
Evaluation framework
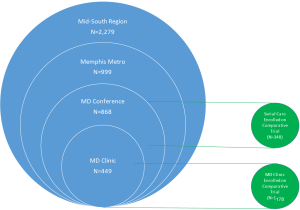
We used the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) (17) framework to evaluate key aspects of the program’s impact. This report is limited to the evaluation of the Reach and Implementation domains of RE-AIM. To evaluate the generalizability of the multidisciplinary patient population (Reach), we considered 4 concentric layers of lung cancer patients (Figure 1), to compare our patient population to local and regional patient populations: the multidisciplinary clinic, multidisciplinary conference, the Memphis Metropolitan Area lung cancer population, and the Mid-South (Eastern Arkansas, North Mississippi, Western Tennessee) regional healthcare system lung cancer population. Data sources for groups 1 and 2 were collected specifically as part of our study and included all patients seen at the multidisciplinary clinic or discussed at multidisciplinary conference from 2014–2016, regardless of participation in the comparative effectiveness aims. Data for groups 3 and 4 were obtained from the healthcare system’s Cancer Registry [2014–2015]. These results are descriptive, and are not compared statistically because they do not represent independent groups of patients.
Results
In phase 1, we solicited stakeholder feedback using a combination of focus groups and in-depth interviews. Details have been reported elsewhere (10,11,18).
Phase 2: implementing the multidisciplinary clinic
We used the stakeholder feedback to inform the structure and processes of the clinic and to develop objective performance benchmarks representative of the spectrum of stakeholder perspectives.
Implementation benchmarks and the targeted goals
The multidisciplinary clinic obtained an overall treatment concordance rate of 90.3% compared to a target rate of >85% (Table 1). Satisfaction scores were obtained from 93% of patients, 97% of caregivers, and 50% of providers (target 60%). More than 95% of both patients and caregivers rated themselves as being “very satisfied” with all aspects of care received from the multidisciplinary team, including communication about the care received, having all of their questions answered, and the staff working well together. Both patients and caregivers were also very satisfied with the treatment planning, with more than 97% reporting that they ‘left the appointment with a clear understanding of the recommended next steps’ (target 80%). Finally, verifiable communication within two days with patients, caregivers and all providers connected with each patient was achieved in 90.6% of patient clinic visits (target >80%).

Full table
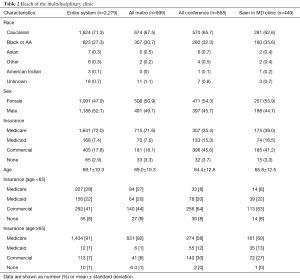
Reach: patient characteristics
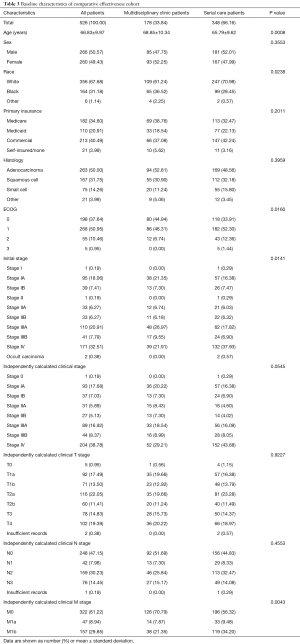
We evaluated the Reach of the multidisciplinary clinic by comparing patient characteristics across four sequentially broader comparison groups (Table 2). The racial distribution of all persons seen in the multidisciplinary clinic (regardless of participation in the comparative effectiveness study), was skewed towards a higher proportion of black patients, than all conference, metropolitan area, or the entire regional healthcare system. Multidisciplinary clinic patients had a similar sex distribution and mean age to all conference patients, which appeared younger, with more female patients than each of the broader comparative groups. The distribution of insurance type indicated more multidisciplinary clinic patients had commercial insurance than comparative groups other than the conference group. This effect was attenuated to some degree after stratifying by age (<65 vs. ≥65).
Full table
Phase 3: comparative effectiveness study
Patient enrollment
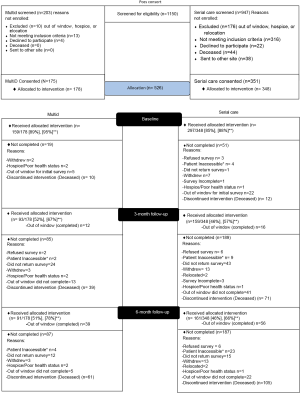
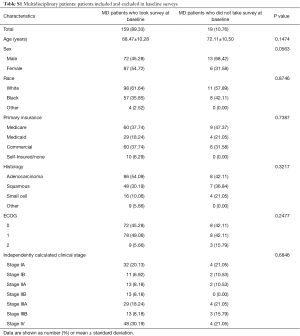
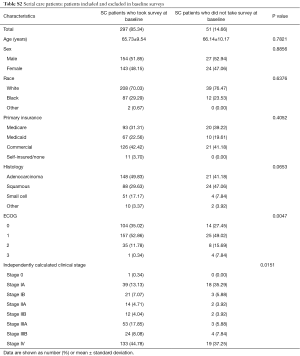
A total of 203 patients were screened for the multidisciplinary clinic arm of the comparative effectiveness trial. Of these, 10 (5%) were unable to participate because they were out of the time window, in hospice care, or relocated; 13 (6%) did not meet the inclusion criteria; 4 (2%) declined to participate; the reason for non-enrollment of 1 patient was not recorded. In total 178 multidisciplinary care patients consented for the study, including 175 of those screened for the multidisciplinary arm plus 3 who were screened for serial care but crossed over to multidisciplinary care after consent but before initiating the study (Figure 2). Of the 178 multidisciplinary patients who consented, 159 (89%) responded to the baseline survey. Multidisciplinary care patients who did not complete the baseline survey did not differ significantly in sex, race, age, ECOG performance status, or clinical stage compared to those who completed the survey (Table S1).
Full table
In the serial care arm, 947 patients were screened. Of these 176 (19%) were unable to participate because they were out of the time window, in hospice care, or relocated; 316 (33%) did not meet the inclusion criteria, 22 (2%) declined to participate, 44 (5%) were deceased, 38 (4%) sent to another site (Figure 2). In total 351 (37%) of the screened patients consented, and 3 subsequently crossed-over to the multidisciplinary care arm, leaving 348. Of the 348, 297 (85%) responded to the baseline survey. Serial care patients who did not complete the baseline survey had significantly higher ECOG score, lower clinical stage, similar sex, similar race, and similar age distributions compared to those who did complete the survey (Table S2).
Full table
Patient characteristics
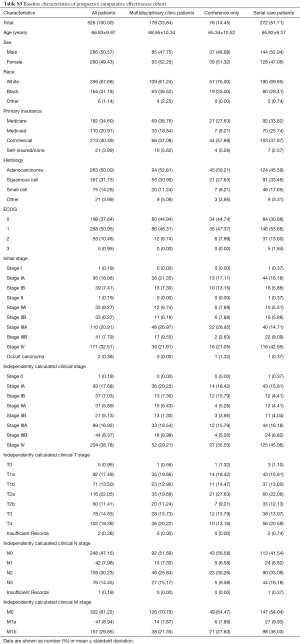
Patients on the multidisciplinary care arm of the comparative effectiveness study had a mean age of 69 and 37% were privately insured, compared to a mean age of 66 and 41% privately insured in the broader multidisciplinary population used for evaluating Reach (Tables 2,3). The serial care patients in the comparative effectiveness study had a lower mean age and a higher percentage of private insurance compared with the metropolitan area and the entire healthcare system, but the distributions of race and sex were reasonably close (Tables 2,3).
Full table
In comparison to the serial care patients, multidisciplinary clinic patients were older (average age: 69 vs. 66 years old, P=0.0008) and were a larger percentage minority race (P=0.0238, Table 3). The distributions of sex and insurance were similar between the two arms. ECOG performance status was significantly better (lower) in multidisciplinary patients compared with serial care (ECOG 0/1/2: 45%/48%/7% vs. 34%/52%/12%; P=0.0160). The initial aggregate clinical stage (defined as clinical stage at the point of initial radiologic detection, before additional workup) distribution was higher in the serial care arm (P=0.0141), driven by the higher numbers of clinical M1 patients. The difference in final clinical stage (clinical stage at the point just before treatment onset, after workup is completed) trended towards significance (P=0.0545), also driven by a significant difference in the M category distribution (P=0.0043). The distributions of T- and N-category were similar. Thus, of the pre-specified matching parameters, the insurance distribution was similar, and there were some differences in clinical stage, performance status, race, and age (Table 3). The risk-set matching resulted in 32 risk strata ranging in size from 2 to 43 patients.
For secondary analyses, the serial care arm will be delineated into two groups based on the care received, ‘conference only’ and ‘true’ serial care (Table S3). Of the 348 serial care patients, 76 were discussed in the multidisciplinary conference (‘conference only’). These patients had similar sex, race, and age distributions compared to true serial care patients. However, conference only patients were 58% commercially insured and 45% had ECOG score of 0, compared with 38% and 31% in true serial care. Future secondary analyses for this study will evaluate the conference only subset as a separate group.
Full table
Baseline health-related quality of life
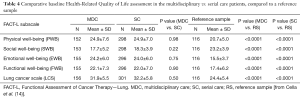
Results from the FACT-L surveys were similar between patients on the multidisciplinary and serial care arms for ‘physical well-being’, ‘social well-being’, ‘emotional well-being’, ‘functional well-being’, and ‘lung cancer specific quality of life’ (all P>0.10, Table 4). Patients in both arms of our study had significantly higher scores than published reference data for ‘physical well-being’, ‘emotional well-being’, ‘functional well-being’, and ‘lung cancer specific quality of life’, and lower scores for ‘social well-being’ (all P<0.0001, Table 4).
Full table
Discussion
We demonstrate a highly successful implementation of a multidisciplinary care-delivery model for lung cancer patients in a non-academic community health care system within a high lung cancer incidence and mortality region of the United States. Implementation benchmarks established by stakeholders were uniformly attained. The multidisciplinary program successfully reached a higher percentage of minority patients than regional comparison groups, and the average patient age was lower.
The comparative effectiveness trial conducted in the last phase of the study met the planned enrollment and the sample size per statistical design. Patients on the multidisciplinary arm of this trial were older and less likely to be privately insured than the broader multidisciplinary clinic population. The serial care patients were younger and more likely to be privately insured compared to the regional population. Therefore, the distribution of age and race differs between the two comparative effectiveness arms, with higher age and a higher percentage minority on the multidisciplinary arm. Serial care patients also had higher stage than the multidisciplinary arm, driven by the percentage of patients with metastatic disease.
The differences between the two comparative effectiveness trial arms are not unexpected in a pragmatic trial, and will be adjusted for by statistical modeling, per study design. Results from the multidisciplinary clinic are generally representative, but differences in race and age distributions should be acknowledged when generalizing our findings. The higher percentage of patients on the serial care arm with metastatic disease may impact the unadjusted survival distribution; however, the risk-stratified models will adjust for this difference. Additional analyses will also be conducted excluding all patients with metastatic disease to balance the stage distribution between arms. However, it is also important to recognize that thoroughness of staging was a process end-point in this study, including the rate of tissue confirmation of stage, up-staging and down-staging. It is therefore likely that some of the apparent stage asymmetry results from intervention-induced differences.
While the study design has high external validity, the non-randomized nature of this trial is a potential limitation to its internal validity (19). Due to the type of intervention we implemented and the pragmatic approach of our study we could not randomly assign patients to treatment groups, leading to potential imbalance. Furthermore, we were unable to fully execute the planned prospective matching strategy because of the limited availability of serial care participants at the outset of accrual. We have structured the analyses to control for these imbalances. Additionally, we were not able to directly compare cost-effectiveness between the models of care because of restrictions imposed by the funding organization.
Strengths of this study include the prospective study design, which incorporated a structured and detailed collection of pre-specified clinical data elements. Secondly, the multi-stakeholder-guided initial phase helped identify relevant benchmarks and endpoints, making this not only a patient-centered, but also a multi-stakeholder-centered study. Third, the team science approach allowed for input from clinicians and scientists from a variety of disciplines. Fourth, the mixed-methods approach combined rigorously structured quantitative data with the contextually rich and detailed information from qualitative evaluations. Additionally, we were able to plan the implementation study during the initiation of the multidisciplinary clinic, which allowed for maximum flexibility in structuring the clinic.
Conclusions
This study demonstrates a comprehensive implementation of a multidisciplinary model of care in lung cancer, which will advance the science behind implementing this much-advocated clinical care model. We will report the analysis of the adoption, effectiveness and maintenance domains of RE-AIM in due course, including the comparison of process endpoints and outcomes, including survival. We thereby hope to quantify the value of multidisciplinary care, while concurrently providing generalizable implementation know-how that can stimulate future wider dissemination.
Acknowledgements
The authors would like to thank all patients and their caregivers, clinicians, hospital administrators and insurance company executives who gave advice and participated in the various components of the study.
Funding: IH-1304-6147. PCORI. (PI: Raymond Osarogiagbon, MBBS). Building a multidisciplinary bridge across the quality chasm in thoracic oncology. Patient Center Outcomes Research Institute (PCORI)
Footnote
Conflicts of Interest: Dr. R Osarogiagbon has the following potential conflicts of interest: stock ownership in Eli Lilly, Foundation Medicine and Pfizer; paid consultant for Association of Community Cancer Centers, Eli Lilly, Roche/Genentech; paid speaker for Roche/Genentech; patent application pending for a surgical lymph node specimen collection kit. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Baptist Memorial Healthcare Corporation Institutional Review Board (IRB) and the University of Memphis IRB (ID: 3385). All participants in the qualitative studies, and patients and caregivers in the comparative effectiveness study provided written informed consent.
References
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016.
- Little AG, Gay EG, Gaspar LE, et al. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer 2007;57:253-60. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Osarogiagbon RU. Overcoming the implementation gap in multidisciplinary oncology care programs. J Oncol Pract 2016;12:888-91. [Crossref] [PubMed]
- Osarogiagbon RU, Rodriguez HP, Hicks D, et al. Deploying team science principles to optimize interdisciplinary lung cancer care delivery: Avoiding the long and winding road to optimal care. J Oncol Pract 2016;12:983-91. [Crossref] [PubMed]
- National Comprehensive Cancer Network clinical practice guidelines in oncology. Non-small cell lung cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed July 17, 2017.
- National Comprehensive Cancer Network clinical practice guidelines in oncology. Small cell lung cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed July 17, 2017.
- ASCO-ESMO Consensus Statement on Quality Cancer Care. J Clin Oncol 2006;24:3498-99. [Crossref] [PubMed]
- Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. Washginton, DC: The National Academies Press, 2013.
- Kedia SK, Ward KD, Digney SA, et al. 'One-stop shop': lung cancer patients' and caregivers' perceptions of multidisciplinary care in a community healthcare setting. Transl Lung Cancer Res 2015;4:456-64. [PubMed]
- Kedia S, Ward KD, Jackson B, et al. Bridging the quality chasm in lung cancer care: Stakeholder perspectives on multidisciplinary care in a community hospital setting. IASLC 16th World Conference on Lung Cancer 2015; ORAL27.01 Denver, CO, USA.
- Annunziata MA, Muzzatti B, Altoè G. Defining hospital anxiety and depression scale (HADS) structure by confirmatory factor analysis: a contribution to validation for oncological settings. Ann Oncol 2011;22:2330-3. [Crossref] [PubMed]
- Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol 2004;22:2992-6. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy – Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Farjah F, Flum DR, Ramsey SD, et al. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol 2009;4:355-63. [Crossref] [PubMed]
- Dzewaltowski DA, Glasgow RE, Klesges LM, et al. RE-AIM: Evidence-based standards and a web resource to improve translation of research into practice. Ann Behav Med 2004;28:75-80. [Crossref] [PubMed]
- Kedia SK, Ward KD, Digney SA, et al. A qualitative assessment of organizational barriers to optimal lung cancer care. J Community and Supportive Oncol 2017. In press.
- Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research. Issues in external validation and translation methodology. Eval Health Prof 2006;29:126-53. [Crossref] [PubMed]


