Do EGFR tyrosine kinase inhibitors (TKIs) still have a role in EGFR wild-type pre-treated advanced non-small cell lung cancer (NSCLC)?—the shifting paradigm of therapeutics
Introduction
EGFR mutations are well established as important oncogenic drivers that occur in 10–44% of primary lung adenocarcinomas occurring more frequently in women, Asians and non-smokers (1,2).
Gefitinib and erlotinib, tyrosine kinase inhibitors (TKIs), were developed and first applied clinically before the significance of EGFR mutations was established, in the era when lung cancer therapy was largely empirical. A link between the presence of activating EGFR mutations and sensitivity to gefitinib was established in 2004 in a phenotypically enriched population (1,2), however it was still several years before large randomized controlled trials (RCTs) confirmed the superiority of a “targeted” drug approach in patients with sensitizing EGFR mutations over standard chemotherapy. In this time, RCTs were also conducted evaluating the empirical use of erlotinib and gefitinib versus placebo as second or third line therapy in unselected patients with chemotherapy refractory non-small cell lung cancer (NSCLC) and in other clinical settings such as maintenance therapy. Despite modest benefits when used empirically in unselected patients, the dramatic effects of these agents in patients with sensitizing EGFR mutations redefined modern practice as it is today. Molecular selection for EGFR mutations and anaplastic lymphoma kinase (ALK) gene rearrangements is now standard practice in advanced NSCLC while more generalised molecular characterisation is becoming increasingly popular. Patients lacking sensitizing EGFR mutations [EGFR wild-type (EGFR-wt) NSCLC] continue to be treated based on traditional empirical chemotherapy evidence.
In the era of immunotherapy and the advancing molecular landscape, there is a need to reassess the role of EGFR TKIs in EGFR-wt NSCLC.
Background for TKIs in EGFR-wt NSCLC
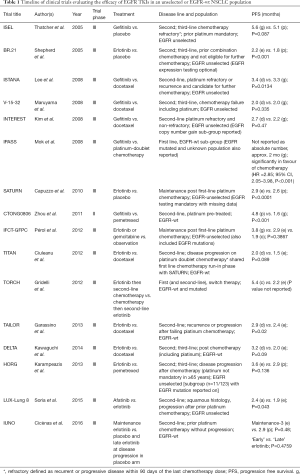
The empirical use of EGFR TKIs compared to placebo in treatment refractory second and third line unselected NSCLC patients was reported in the ISEL trial in 2005, with no improvement in survival observed with gefitinib (3), while statistically significantly improved survival was seen with erlotinib in the same year (4). However, these study populations were not thoroughly assessed for the presence of EGFR mutations. Thus, they can not be considered as EGFR-wt populations as the true rate of EGFR mutations, which may have biased the observed clinical benefits, was not known (Table 1).
Full table
In the large BR.21 trial, 731 patients were randomized to erlotinib or placebo in the second and third line settling. All NSCLC histologies were included and EGFR testing was not required for enrolment. Overall survival (OS) favoured erlotinib [hazard ratio (HR) =0.73; 95% confidence interval (CI), 0.49–1.1]; however, ongoing treatment if any was not described (4). In further investigation, EGFR mutation testing was successful in 197 tumour specimens with a mutation identified in 40 patients (20.3%). Mutational status had no statistically significant association with objective response rate (ORR): 7% EGFR-wt patients achieved a response, compared with 16% of those with an EGFR mutation (P=0.37) (4,5). Furthermore, erlotinib resulted in an absolute improvement over placebo in progression free survival (PFS) of 0.4 months. Based on this trial, erlotinib was widely used in Europe, the USA and elsewhere as empirical therapy in refractory NSCLC patients.
In 2010 the phase III SATURN study evaluated erlotinib or placebo in 889 patients with NSCLC in the first-line switch maintenance setting. The study included patients with stable or responsive disease after standard first line chemotherapy. Although PFS was reported as ‘significantly’ longer with erlotinib than with placebo, the difference in median PFS was modest 12.3 weeks for patients on erlotinib versus 11.1 weeks placebo (HR =0.71; 95% CI, 0.62–0.82 weeks; P<0.0001). Both the adenocarcinoma and squamous (SCC) sub-populations derived benefit. ORR was 11.9% with erlotinib versus 5.4% with placebo and OS reported significantly longer by 1 month (HR =0.77; 95% CI, 0.64–0.93 months, P=0.0063). Forty-four percent were EGFR-wt, 6% found to have an EGFR mutation, and the remainder having a missing or indeterminate result (6). The IFCT-GFPC phase III study in 2012 investigated whether continuation maintenance with gemcitabine or switch maintenance with erlotinib improved clinical outcomes compared with observation in patients with unselected NSCLC whose disease was controlled after cisplatin-gemcitabine induction chemotherapy. Both gemcitabine and erlotinib prolonged PFS versus observation with improvements in the median PFS by a modest 1.9 and 1.0 months respectively (7).
Moving back, between 2008 and 2010 three RCTs compared gefitinib with docetaxel in unselected patients with previously treated advanced NSCLC. In a Korean population, the ISTANA trial reported improved OS, and quality of life with gefitinib but inferior PFS. The prevalence of EGFR mutations has been reported as 20% in Korean patients, although it was not defined in this trial (8). In the Japanese V-15-32 study there was no difference between gefitinib and docetaxel with regard to OS or PFS (9). The international INTEREST trial demonstrated non-inferiority with gefitinib versus docetaxel in a large study cohort, including patients with copy number gain in the EGFR gene (10).
The efficacy and safety of pemetrexed or gefitinib as second-line treatments for advanced EGFR-wt non-squamous NSCLC was investigated in the randomized phase II CTONG0806 trial in Chinese patients, reported in 2011. Pemetrexed showed significant improvement in PFS compared with gefitinib (4.8 vs. 1.6 months, P<0.001) (11).
In the TAILOR and DELTA trials of 2013 and 2014 respectively, erlotinib failed to improve OS in comparison with docetaxel in EGFR-wt cohorts (12,13), whereas erlotinib and pemetrexed demonstrated similar OS (P=0.986) in a third HORG phase III trial unselected for EGFR (14).
In the 2012 TITAN trial erlotinib was compared with chemotherapy in a population of EGFR-wt NSCLC patients with poor prognosis and progressive disease during or immediately after first-line chemotherapy. This trial showed no significant difference in OS between the two groups either (5.3 vs. 5.5 months, respectively; HR =0.96; 95% CI, 0.78–1.19; log-rank P=0.73) however the safety profile again favoured erlotinib (15).
Aforementioned, these trials largely tested EGFR TKIs in unselected patients, and rapid and durable responses were more often observed in patients phenotypically enriched for the presence of EGFR mutations; adenocarcinoma histology, Asian ethnicity, and a history of never or light smoking. In the landmark 2008 Iressa Pan-Asia Study (IPASS) however, among patients with EGFR-wt NSCLC, gefitinib compared to carboplatin and paclitaxel in the first line setting resulted in a significantly inferior ORR (1.1% vs. 23.5%, P=0.001) and shorter PFS (HR =2.85; 95% CI, 2.05–3.98; median PFS 1.5 and 5.5 months, respectively) (16).
In 2012 subsequent to IPASS, again in the first line setting, the phase III TORCH trial investigated erlotinib followed by cisplatin and gemcitabine at the time of disease progression compared to the standard sequence of cisplatin and gemcitabine followed by erlotinib at the time of disease progression in unselected patients. In the subgroup of patients with EGFR-wt NSCLC (n=236), erlotinib followed by cisplatin and gemcitabine compared to the inverse sequence was associated with inferior OS (HR =1.29; 95% CI, 1.58 to 2.71; median, 6.5 and 9.8 months, respectively) and inferior PFS (17).
In 2015 the LUX-Lung 8 study, which compared the second generation EGFR TKI afatinib versus erlotinib in pre-treated patients with SCC, a small PFS benefit was observed favouring afatinib (median PFS 2.4 vs. 1.9 months respectively, HR =0.82; P=0.043) (18). There was no chemotherapy or observation control arm in this trial.
Finally, in 2016 the phase III IUNO trial re-assessed the benefit of maintenance erlotinib versus erlotinib at progression in advanced/metastatic NSCLC that had not progressed following four cycles of platinum-based chemotherapy. Median OS was 9.7 and 9.5 months with ‘early erlotinib’ and ‘late erlotinib’, respectively (P=0.82). OS with maintenance erlotinib was not superior to second-line treatment in EGFR-wt patients. Maintenance treatment with erlotinib in patients with advanced/metastatic NSCLC without EGFR-activating mutations was concluded to be considered unfavourable (19) (Table 1).
Article summary
The Intergroupe Francophone de Cancérologie Thoracique (IFCT) Biomarkers France study undertook nationwide genetic tumour profiling in patients with NSCLC, demonstrating the utility of this approach in directing the most suitable therapeutic sequence. This large database was utilised to enable the trial under-review in this editorial (20).
In 2017, Tomasini et al. reported on this retrospective real-world study that recruited patients with advanced, largely non-squamous NSCLC who completed molecular testing by the 28 French National Cancer Institute accredited centres between April 2012 and April 2013. Patients were eligible if they did not harbour an EGFR mutation or ALK rearrangement. The molecular panel funded for routine testing included EGFR, KRAS, BRAF, PI3KCA mutations and ALK rearrangement. All patients who had received prior first line chemotherapy and second line chemotherapy at the time of progression were eligible and clinical outcome data were provided by the prescribing physician (21).
The primary outcome of interest from this dataset was second-line PFS and OS.
Data were cut June 1, 2015 and 17,640 NSCLC patients for whom molecular testing had been undertaken were potentially eligible with 1,278 patients eventually included after meeting inclusion criteria. Altogether, 410 patients were treated with a second-line EGFR TKI and 868 second-line chemotherapy. The median follow-up time was 11.4 (range, 10.3–12.4) months. There were more male patients included (68.8%) than female (32.1%).
Potentially confounding baseline characteristics include more non-smokers in the EGFR TKI group than in the chemotherapy group (16.7% vs. 8.8%, respectively; P<0.001) and fewer patients with KRAS-mutated tumours (24.9% vs. 33.8%; P=0.001). There were more patients with ECOG performance status ≥2 and more elderly patients (≥65 years) in the EGFR TKI group than in the chemotherapy group (27.1% vs. 18.2%; P=0.001 and 46.8% vs. 32.7%; P<0.001, respectively).
After adjusting for differences in observed characteristics between treatment groups, median OS and PFS in patients treated with chemotherapy were longer than those with EGFR TKI: OS 8.4 vs. 5.0 months, respectively (HR =0.70; 95% CI, 0.6–0.8 months; P<0.0001) and PFS 4.3 (3.88–4.83) vs. 2.8 (2.6–3.1) months, respectively (HR =0.66; 95% CI, 0.57–0.77 months; P<0.0001).
In multivariate analyses response to first-line chemotherapy (P<0.001) and smoking status (P<0.001) were prognostic factors predicting a longer OS with EGFR TKI.
PFS and OS of EGFR-wt patients treated with second-line EGFR TKI were inferior to those encountered in patients receiving second-line chemotherapy, even when corrected for potential confounding characteristics.
Noticeably, in both groups of patients, those who derived a substantial benefit from first-line therapy also benefited from second-line therapy independent of what the second-line regimen was.
In trials comparing second-line EGFR TKI with either placebo or chemotherapy aforementioned, in patients with EGFR-wt tumours, KRAS status was often not routinely assessed therefore any potential imbalance between therapeutic arms with respect to KRAS-activating mutations unknown. In BR.21, INTEREST and TAILOR, KRAS mutated patients did not benefit from erlotinib (5,10,14). Non-smoking patients, KRAS-wt treated with EGFR TKIs derived a meaningful OS improvement in this study (HR =0.43, 95% CI, 0.28–0.66; P<0.001); however, numbers may have been small and were not reported (21).
Current EGFR-wt TKI guidelines
In 2017, the National Comprehensive Cancer Network (NCCN) panel deleted the recommendation for erlotinib as switch maintenance therapy and as subsequent therapy in non-squamous wild-type NSCLC (22). This was based on preliminary results from the randomized IUNO trial and revised indication by the U.S. Food and Drug Administration (FDA) (19,23). The data showed that survival was not improved with erlotinib versus placebo.
On October 18, 2016, the U.S. Food and Drug Administration modified the indication for erlotinib for treatment of NSCLC to limit use to patients whose tumours have specific EGFR mutations (23).
The 2016 European Society of Medical Oncology (ESMO) also updated their guidelines to remove erlotinib as switch maintenance therapy in NSCLC, however recommend erlotinib still represents a potential second/third-line treatment option in pre-treated patients with unknown or EGFR-wt status based on limited efficacy compared with chemotherapy [II, C] (24).
Immunotherapy
This trial recruited prior to immunotherapy being readily available second-line outside of a clinical trial. The first phase III trial demonstrating the superior efficacy of program death receptor-1 (PD-1) inhibitor nivolumab over docetaxel chemotherapy was reported in July 2015 (25); Checkmate-017 demonstrated a 3.2 month OS benefit in squamous NSCLC, further supported by Checkmate-057 with a 2.8 month survival benefit (26). Ongoing comparable data to support the efficacy of PD-1 inhibitor pembrolizumab and program death ligand-1 (PD-L1) inhibitor atezolizumab have followed and changed our practice and the landscape of treating advanced NSCLC as further compelling data emerge in the first line setting and in combination therapy (27,28).
Molecular therapy in oncogene addicted NSCLC
In the last 10 years, in part enabled by the availability of advancing diagnostics with next generation sequencing, personalized therapy has evolved exponentially as a number of oncogenes in NSCLC have been described, with differing characteristics, therapeutics and prognostic implications.
A platform for personalized medicine has developed, with many clinical trials investigating and reporting on the efficacy of treatment not only for EGFR mutant NSCLC, but ALK, ROS1, MET rearranged, MET exon 14 skipping mutation positive, NTRK fusion positive, BRAF and HER2 mutant, RET amplified and KRAS mutant NSCLC. Most of these gene signatures were not known or reported in the EGFR TKI literature reviewed here.
The NCI-Match Trial (Molecular Analysis for Therapy Choice) is an example of a precision medicine treatment clinical trial in which patients are assigned to receive treatment based on the genetic changes found in their tumours through genomic sequencing (29).
Conclusions
Given the current landscape of treatment for metastatic NSCLC, and advances into the era of empirical immunotherapy and molecular therapeutics, there is little enthusiasm nor rationale for the further investigation of EGFR TKI therapy alone or in combination in EGFR-wt NSCLC.
Furthermore, the definition of ‘EGFR-wt’ has certainly evolved in recent years, so the past literature must be interpreted cautiously, understanding that study data have come from heterogeneous patient populations largely predating reflex modern molecular testing for the presence of an EGFR mutation or more complex molecular panels used widely today.
The several large scale clinical trials investigating the efficacy of EGFR TKIs used empirically in mostly unselected patients have resulted in some statistically significant results to argue in the past for their use. But with the improved negative patient selection in more recent trials (by using wider molecular profiling to exclude patients with oncogene drivers), and the safety and substantial clinical efficacy observed with immunotherapy in these better defined populations for empirical therapy; the clinical significance of the modest benefits seen with EGFR TKIs in this setting must be questioned. Further investigation in to this issue is not of clinical interest nor relevant in the current lung cancer treatment paradigm.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med 2005;353:133-44. [Crossref] [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3516-24. [Crossref] [PubMed]
- Lee DH, Park K, Kim JH, et al. Randomized phase III trial of gefitinib versus docetaxel in non–small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307-14. [Crossref] [PubMed]
- Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. [Crossref] [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [Crossref] [PubMed]
- Zhou Q, Cheng Y, Yang JJ, et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol 2014;25:2385-91. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second-or third-line therapy in patients with advanced non–small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902-8. [Crossref] [PubMed]
- Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non–small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013;119:2754-64. [Crossref] [PubMed]
- Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300-8. [Crossref] [PubMed]
- Mok T, Wu YL, Thongprasert S, et al. Phase III, randomized, open-label, first-line study of gefitinib (G) versus carboplatin/paclitaxel (C/P) in clinically selected patients (pts) with advanced non-small-cell lung cancer (NSCLC). Ann Oncol 2008;19:viii1-4.
- Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non–small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002-11. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study). Lung Cancer 2016;102:30-7. [Crossref] [PubMed]
- Morin F, Lebitasy MP, Tran Q, et al. L’Intergroupe Francophone de Cancérologie Thoracique (IFCT) Bilan des 10 premières années. Revue de Pneumologie Clinique 2009;65:S42-8. [Crossref]
- Tomasini P, Brosseau S, Mazières J, et al. EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR wild-type pre-treated advanced nonsmall cell lung cancer in daily practice. Eur Respir J 2017;50:1700514. [Crossref] [PubMed]
- NCCN. Non-small Cell Lung Cancer Version 1.2018-November 17, 2017.
- Erlotinib (Tarceva). Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525739.htm
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Institute NC. NCI-MATCH Trial (Molecular Analysis for Therapy Choice). US Department of Health and Human Services 2017.


