An alternative way to initiate Notch1 signaling in non-small cell lung cancer
Notch signaling has a critical role in regulating cross-talk between cells and regulates a variety of processes, including cell fate determination, cell proliferation, and death. The mammalian Notch receptor family consists of four type I transmembrane receptors (termed Notch1-4). Notch signaling is usually initiated when a Notch ligand attaches to a Notch receptor, a process mediated by cell-to-cell contact. To date, there are five known Notch ligands in mammals, including jagged proteins (JAG1, JAG2) and Delta-like proteins (Dll1, Dll3, Dll4). The primary role for the Notch ligands on signal-sending cells is to initiate Notch signaling in signal-receiving cells by triggering the proteolytic cascade of Notch receptors and releasing the active intracellular domain of Notch ligand (NICD) from the plasma membrane. Once the active NICD is translocated into the nucleus, it mediates transcriptional activation of downstream genes, including those in the Hairy Enhancer of Split (HES) family (Figure 1A). In cancer cells, Notch signaling can be active alone, or in cross-talk with other signaling pathways, including Wnt and TGF-β (1,2). Moreover, activated Notch receptors on endothelial cells (ECs) were shown to negatively regulate the expression of VEGFR2, a VEGF receptor, in the same cells (3). Together, these finding suggested that the regulation of Notch signaling in cancer cells is comprehensive and not fully understood. Notch signaling is usually not activating unless the Notch receptor contacts one of its ligands (Figure 1B); however, gain-of-function Notch1 mutations were reported in 10% of clinical NSCLC cases (4). In these tumor cells, Notch1 is constitutively active without engagement of any Notch ligand to promote survival (5) (Figure 1C).
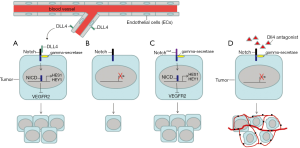
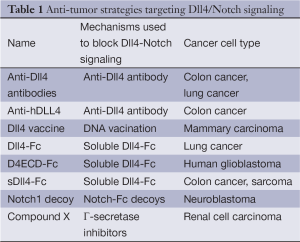
As an endothelium-specific Notch ligand, Dll4 is strongly upregulated in tumor vasculature in mouse models and in many human cancers, including kidney, bladder, colon, brain, breast and gastric cancers (6,7). In clinical studies, upregulation of Dll4 is associated with poor clinical outcomes in many human cancers (7,8), and increased risk of lymph node metastases (7). In preclinical studies of cancer treatment, Dll4 has been identified as a promising new target in tumor angiogenesis and inhibiting Dll4/Notch signaling significantly suppressed tumor growth (6) (Table 1). Agents disrupting Dll4-Notch signaling can reduce tumor growth in two possible ways. First, antagonists can affect survival of tumor-initiating stem cells (9). Second, antagonists increase the number of endothelial tip cells in the tumor to form immature angiogenic vessels. These vessels are not well perfused and are poorly functional, reducing the effective blood flow in the tumor to suppress cancer cell growth (10) (Figure 1D). Pharmacological Dll4 inhibitors are now entering clinical trials for solid tumors. Demcizumab (OMP21M18), a humanized anti-Dll4 monoclonal antibody, alone or in combination with other drugs is currently in two phase 1 studies in solid tumors, three Phase 1b studies in non-small cell lung cancer (NSCLC), pancreatic cancer and colorectal cancer, and one Phase 1b/2 study in epithelial ovarian cancer (11).
Full table
Studies of Dll4-induced Notch activation in cancer cells mainly focus on understanding Dll4 interaction with the Notch1 receptor, and interruption of Dll4/Notch1 signaling has become a target of therapeutic development. Dll4/Notch3 also promotes the growth of T-lineage acute lymphoblastic leukemia (T-ALL) and colorectal cancer (12). Notch1 promotes the survival of NSCLC cells (13,14) and upregulation of Notch1 was reported in 30% of NSCLC cases (15). Notch3 plays an oncogenic role in lung cancer and its upregulation was reported in 40% of NSCLC (16). The specific role of Notch2 and Notch4 in NSCLC remains unclear (17).
Recently, a paper entitled “Cross-talk between ECs and tumor via delta-like ligand4/Notch/PTEN signaling inhibits lung cancer growth” was published in Oncogene in 2012. In this issue, Ding et al. proposed that endothelial Dll4 triggers Notch1 signaling activation in surrounding NSCLC cells to suppress cancer cell growth (18). Proliferation of NSCLC in vitro and in a xenograft mouse model was induced when co-cultured with human umbilical vein ECs, which express endothelial Dll4 to activate Notch signaling in NSCLC cells (Figure 2A). The researchers further demonstrated that the proliferation of NSCLC cells was reduced when co-cultured with ECs that overexpressed Dll4 (Figure 2B). In a xenograft mouse model harboring a mixed population of Dll4-expressing human ECs and NSCLC cells, tumor weight and tumor volume were both decreased. In addition, NSCLC proliferation was increased when co-cultured with the human ECs that did not express Dll4 (Figure 2C), and the tumor sizes and volumes were greater than the control group. To explore the possible mechanisms mediating NSCLC proliferation through Dll4/Notch signaling, Ding et al. isolated the NSCLC cells that were co-cultured with Dll4-expressing human ECs and showed that transcriptional expressions of Notch1, HES1, and HEY1 were elevated in the cancer cells. Increased expression of Notch1 and downstream genes in the xenograft tumors indicates that Notch signaling is activated in these tumor cells. Contrary to the previous finding that Notch1 activation decreased PTEN expression in NSCLC (13), Ding et al. showed that PTEN expression was elevated in the NSCLC cells co-cultured with Dll4-expressing ECs, implying that the PI3K/AKT pathway was suppressed in these NSCLC cells (Figure 2B). To confirm this finding, additional demonstration of the suppression of PI3K/AKT signaling in these NSCLC cells and their contribution to tumor suppression would be necessary. Because Notch regulation in cancer cells is complex, the elevation of PTEN during Notch1 activation in these NSCLC may be context-dependent.
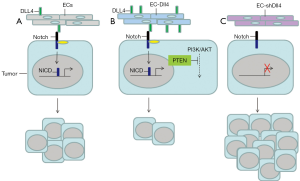
Ding et al. reported an intriguing observation that co-culturing with human ECs lacking Dll4 expression induced NSCLC cell proliferation in vitro. In addition, tumor weight and tumor volume increased in xenograft mice injected with ECs lacking Dll4, when compared to control mice. Moreover, transcriptional and translational expressions of Notch1 were both decreased in NSCLC cells when co-cultured with Dll4-overexpressing ECs. Several compelling questions arise from these findings (18). First, Notch1 signaling is activated in NSCLC cells (19). How generally is the Notch1 signaling in NSCLC cells influenced by Dll4 from human ECs in the co-cultured system, aside from the Notch1 signaling regulated between NSCLC cells or with other cell types in the xenograft tumors? Dll4 reportedly acts downstream of VEGF in human tumor vasculature (20), suggesting a possible mechanism for inducing Notch1 activation in vivo. Does VEGF induced Dll4 expression contribute to Notch1 activation and cancer cell growth in the xenograft tumors, and if so, how effective is that compared to the Dll4 overexpressed from human ECs in promoting tumor growth? Moreover, because Notch1 is activated in NSCLC cells, it would be crucial to determine how reduction of Notch1 expression in NSCLC cells associates with Dll4 inhibition in the co-cultured human ECs. Finally, the mechanism that induces NSCLC growth when co-cultured with human ECs lacking Dll4 expression was not elucidated by Ding et al. Therefore, studies to understand the mechanisms regulating cancer cell proliferation in this system are warranted. Ding et al.’s work (18), therefore highlights one of the numerous ways that regulate Notch signaling in tumorigenesis.
Acknowledgements
This study was supported by NCI R01 CA140654 (to L.Y.). We are also grateful for support from the Larry Hall and Zygielbaum Family Foundation and Kazan, McClain, Abrams, Fernandez, Lyons, Greenwood, Harley & Oberman Foundation, Inc., the Estate of Robert Griffiths, the Jeffrey and Karen Peterson Family Foundation, the Estate of Norman Mancini, and the Barbara Isackson Lung Cancer Research Fund. Special thanks to Pamela Derish MA, Scientific Publications Manager, UCSF Department of Surgery for editing the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Dai J, Ma D, Zang S, et al. Cross-talk between Notch and EGFR signaling in human breast cancer cells. Cancer Invest 2009;27:533-40. [PubMed]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 2009;19:71-88. [PubMed]
- Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer 2008;99:1204-9. [PubMed]
- Wang NJ, Sanborn Z, Arnett KL, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A 2011;108:17761-6. [PubMed]
- Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis 2013;34:1420-30. [PubMed]
- Kuhnert F, Kirshner JR, Thurston G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc Cell 2011;3:20. [PubMed]
- Ishigami S, Arigami T, Uenosono Y, et al. Clinical implications of DLL4 expression in gastric cancer. J Exp Clin Cancer Res 2013;32:46. [PubMed]
- Jubb AM, Soilleux EJ, Turley H, et al. Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer. Am J Pathol 2010;176:2019-28. [PubMed]
- Hoey T, Yen WC, Axelrod F, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 2009;5:168-77. [PubMed]
- Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 2007;7:327-31. [PubMed]
- Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol Ther 2014;141:140-9. [PubMed]
- Indraccolo S, Minuzzo S, Masiero M, et al. Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Res 2009;69:1314-23. [PubMed]
- Eliasz S, Liang S, Chen Y, et al. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 2010;29:2488-98. [PubMed]
- Chen Y, De Marco MA, Graziani I, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res 2007;67:7954-9. [PubMed]
- Westhoff B, Colaluca IN, D’Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009;106:22293-8. [PubMed]
- Haruki N, Kawaguchi KS, Eichenberger S, et al. Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res 2005;65:3555-61. [PubMed]
- Galluzzo P, Bocchetta M. Notch signaling in lung cancer. Expert Rev Anticancer Ther 2011;11:533-40. [PubMed]
- Ding XY, Ding J, Wu K, et al. Cross-talk between endothelial cells and tumor via delta-like ligand 4/Notch/PTEN signaling inhibits lung cancer growth. Oncogene 2012;31:2899-906. [PubMed]
- Zhang S, Long H, Yang YL, et al. Inhibition of CK2alpha down-regulates Notch1 signalling in lung cancer cells. J Cell Mol Med 2013;17:854-62. [PubMed]
- Patel NS, Li JL, Generali D, et al. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 2005;65:8690-7. [PubMed]


