Durvalumab for non-resectable stage IIIB non-small cell lung cancer—a small step or a big leap?
Over 70% of all non-small cell lung cancer (NSCLC) patients present with advanced or metastatic tumours at the time of diagnosis. Locally advanced (stage III) NSCLC is an important and controversial treatment subgroup comprising 25–30% of all NSCLC at diagnosis (1). Within this patient population, there is a considerable heterogeneity in both, tumour burden and the extent of lymph node involvement. As a result, to date there is no generally accepted therapeutic approach so far and clinical research is ongoing to establish the optimal timing, sequencing and combination of surgery, chemotherapy and radiotherapy for NSCLC patients with locally advanced disease (1).
In contrast to resectable (stage IIIA) NSCLC, the treatment strategy for non-resectable stage III NSCLC using definitive chemo-radiotherapy is well implemented and has a clear clinical rationale. The RTOG 7301 trial was one of the first studies which established 60 Gy to be a potentially curative radiotherapy dose in unresectable NSCLC patients (2), however, local recurrence still remained a major problem, which led to additional trials demonstrating a significant overall suvial (OS) benefit following the addition of induction chemotherapy to definitive radiotherapy (3).
As clearly demonstrated in a recently published meta-analysis of individual patient data, several phase III trials have shown a substantial benefit from treatment with concurrent, rather than sequential, chemo-radiotherapy (4). In addition, for those patients unwilling to undergo or precluded from chemotherapy, accelerated radiotherapy can confer improved outcomes (5). However, median progression-free survival (PFS) among patients who have received chemo-radiotherapy is still poor (approximately 8 months) with only 15% of patients being alive at 5 years (3), and unfortunately no major advances in the treatment of these patients have been made during the last decade (4,6).
One of the initial studies attempting to improve OS of unresectable stage III NSCLC by adding cetuximab (Erbitux®, MerckSerono, Darmstadt, Germany) to a concurrent chemo-radiotherapy was the RTOG 0617 trial (NCT00533949) (7). In this study unresectable stage III NSCLC patients were randomly assigned to receive either 60 Gy (standard dose), 74 Gy (high dose), 60 Gy plus cetuximab, or 74 Gy plus cetuximab. All patients were also treated with concurrent chemotherapy with paclitaxel (45 mg/m2) and carboplatin (AUC 2) weekly. Two weeks after chemo-radiotherapy, two cycles of consolidation chemotherapy separated by 3 weeks were given [paclitaxel (200 mg/m2) and carboplatin (AUC 6)]. Median OS was found to be 28.7 (95% CI, 24.1–36.9) months for patients who received standard-dose radiotherapy and 20.3 (17.7–25.0) months for those who received high-dose radiotherapy [hazard ratio (HR) =1.38, 95% CI, 1.09–1.76; P=0.004). OS in patients who were treated with cetuximab was 25.0 (95% CI, 20.2–30.5) months compared with 24.0 (19.8–28.6) months in those who were not (HR =1.07, 95% CI, 0.84–1.35; P=0.29) (7). From this study it was concluded that 74 Gy radiation given in 2 Gy fractions with concurrent chemotherapy was not better than 60 Gy plus concurrent chemotherapy for patients with unresectable stage III NSCLC and appears to be potentially harmful. Addition of cetuximab to concurrent chemo-radiotherapy and consolidation treatment provided no benefit in OS for these patients (7). In addition, using a slightly different approach, a most recently published smaller phase III trial (n=125) also did not demonstrate an OS difference between induction concurrent radio-chemotherapy followed by consolidation chemotherapy and induction chemotherapy followed by concurrent radio-chemotherapy in patients with unresectable stage III NSCLC (8).
Immuno-oncology is now a well established treatment strategy currently being evaluated in various trials for treatment of NSCLC (9). Of note, this approach differs from current treatment modalities, that attempt to target the tumour directly or are aiming to disrupt the tumour angiogenesis, since it is designed to enhance the patient’s immune response to tumour cells. Immunotherapy is now emerging as a major modality in NSCLC treatment focusing on development of inhibitors of immune checkpoints to boost antitumour immune responses (10). Different immunologic approaches targeting immune checkpoint and co-stimulatory pathways have shown great promise in clinical development with some of them are already approved for first- and second-line treatment of NSCLC (11-13).
Amongst these inhibitors durvalumab (Imfinzi®, AstraZencea, London, UK) is a highly specific human monoclonal antibody (IgG1-kappa) which inhibits the interaction of PD-L1 (programmed cell death ligand-1) with PD-1 (programmed cell death-1) and CD80, but not PD-L2 (14). Currently, a number of ongoing trials in stage IIIB/IV NSCLC patients are attempting to evaluate the role of durvalumab alone and in combination with other checkpoint inhibitors (e.g., tremelimumab, an anti-CTLA-4 monoclonal antibody) or chemotherapy (Table 1).
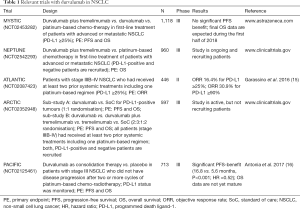
Full table
Most recently, Antonia et al. (16) reported the results of the phase III PACIFIC study (NCT02125461). In this study the role of immune checkpoint blockade with durvalumab in locally advanced, unresectable, stage III NSCLC was evaluated. Eligible patients had NSCLCs without progression after they had been treated with at least two cycles of platinum-based chemotherapy concurrent with radiotherapy (chemo-radiotherapy) at a dose of 54 to 66 Gy. A total of 713 patients were randomly assigned in a 2:1 ratio to receive either the anti-PD-L1 monoclonal antibody durvalumab (10 mg/kg) or placebo every 2 weeks for up to 12 months. A pre-planned interim analysis showed that the co-primary end point of median PFS was 16.8 months in the durvalumab group versus 5.6 months in the placebo group (HR =0.52; 95% CI, 0.42–0.65) (16). In addition, the objective response rate (ORR) (assessed by blinded independent central review) was found to be higher in the durvalumab group than in the placebo group (28.4% vs. 16.0%, P<0.001). Interestingly, clinical benefit was observed irrespectively of NSCLC tumour stage (IIIA or IIIB), histologic type, or geographic distribution. Most notably, however, brain metastases developed far more frequently in the placebo group as in the durvalumab group (11.0% vs. 5.5%) (16,17).
Although the OS data of this study are still not mature, the clinically meaningful PFS difference strongly adds weight to the proposal that durvalumab should be considered as a new standard of care for patients with non-resectable stage IIIB NSCLC. Further evidence for this proposal comes from the observation that the OS benefit frequently exceeds the PFS benefit in other studies of immune checkpoint inhibitors that involve patients with advanced-stage NSCLC (11), and, therefore, seems to be a common finding in immuno-oncology trials.
It should also be noted that in the comparable patient cohort of the RTOG 0617 trial PFS among patients in the control chemo-radiotherapy group was 11.8 months, which was longer than that observed in the placebo group of the PACIFIC study (5.6 months), however, PFS in the RTOG 0617 trial has already been calculated from the initiation of chemo-radiotherapy, whereas in the PACIFIC trial randomisation was performed after patients had completed chemo-radiotherapy (7). Interestingly, the observed PFS benefit was seen in a PD-L1-independent patient population. In the PACIFIC trial patients with a lower tumour PD-L1 expression (<25%) represented a larger proportion of patients trial than patients with a higher PD-L1 expression (≥25%) on tumour cells (16).
PD-L1 expression is extensively evaluated as a biomarker for immunotherapy in NSCLC patients, which has shown some value for predicting response to immuno-oncology drugs targeting the PD-1/PD-L1 axis in some studies, but not in others. The use of PD-L1 as a biomarker remains to be complicated by a number of factors including the variability in tissue collection timing, the antibody and methodology used for staining (including the definition of positivity and the non-standardised test design), the heterogeneity and dynamic of PD-L1 expression within different tumours, and the role of PD-L1 expression on tumour-infiltrating lymphocytes and other immune cells versus the malignant cell population. In addition, it can be speculated that PD-L1 is not just “present” (positive) or “absent” (negative), since it is regarded to be a biological continuum (10,18) and therefore might be of limited value as a biomarker in this subset of patients.
However, the observation that the PFS benefit in the PACIFIC study was found in patients across various histologies (approximately 50% of all patients enrolled had squamous cell NSCLC), in patients with stages IIIA/IIIB NSCLC and also independent of the PD-L1 status, is a novel finding that, if confirmed, may have major clinical implications. On the other hand one can speculate that chemotherapy and radiotherapy may enhance immune-triggered cell death of tumour cells by activating macrophages, dendritic cells and Th1 cells and thereby promoting antigen presentation, resulting in the induction/enhancement of adaptive anti-tumour immune responses (19).
In terms of resectable stage III NSCLC the currently widely accepted treatment concept is based on a number of prospective trials and meta-analyses, however, none of these trials demonstrated a significant difference in OS (1,8,20). In a phase III trial (NCT00002550) patients (n=202) with stage IIIA (N2) NSCLC were randomly assigned in a 1:1 ratio to concurrent induction chemotherapy (two cycles of cisplatin and etoposide) plus radiotherapy (45 Gy) (21). If no progression was observed, patients in group A underwent resection and those in group B continued radiotherapy without any interruption up to 61 Gy. Two additional cycles of cisplatin and etoposide were administered in both groups. Median OS (primary endpoint) was 23.6 months in group A versus 22.2 months in group B (HR =0.87; 95% CI 0.70–1.10; P=0.24). PFS (secondary endpoint) was better in group A than in group B (12.8 vs. 10.5 months, HR =0.77; P=0.017) (20). In a retrospective subgroup analysis the authors reported that OS was improved for patients who underwent lobectomy (but not pneumonectomy), versus chemotherapy plus radiotherapy. From this study it was concluded that chemo-radiotherapy with or without resection (preferably lobectomy) will be an attractive treatment strategies for patients with stage IIIA (N2) NSCLC (21).
In contrast, a closely related trial (ESPATUE trial) Eberhardt et al. (22) reported comparable rates for both, PFS and OS in stage IIIA NSCLC patients treated with induction chemotherapy followed by either surgery or definitive chemo-radiotherapy. Five-year OS was significantly increased in both groups, with 44% of patients treated with resection and 40% of patients treated with definitive chemo-radiotherapy. The study was stopped after 246 of 500 patients had been enrolled due to poor recruitment (22).
Other ongoing immuno-oncology trials are conducted to establish the best treatment with checkpoint inhibitors after surgical resection and may contribute to a better outcome of stage IIIA NSCLC patients. In the phase III PEARLS trials (n=1,380; NCT02504372) patients with stages IB-IIIA NSCLC after resection with or without adjuvant therapy are randomly assigned between placebo and pembrolizumab (anti-PD-1 monoclonal antibody). Primary endpoint is disease-free survival; the study is currently recruiting patients.
The PACIFIC study demonstrated a significant PFS increase with durvalumab irrespective of baseline PD-L1 expression in patients with unresectable stage III NSCLC who had received chemo-radiotherapy, supporting the idea of consolidation treatment in unselected patients. In addition, it is conceivable that durvalumab may be an effective adjuvant therapy in patients with resectable stage III disease after standard treatment, and, at some stage, may terminate the current controversy about the future role of surgery in patients with stage III NSCLC after chemo-radiotherapy. Clearly, this concept will have to be tested prospectively.
In conclusion, the data presented in the PACIFIC trial strongly add weight to the treatment strategy of starting chemo-radiotherapy before inhibition of the PD-1/PD-L1 axis and represent a big leap for treatment of stage III NSCLC. However, refinement of this combined treatment strategy will require further evaluation in terms of timing and duration of the checkpoint inhibitor intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jones CM, Brunelli A, Callister ME, et al. Multimodality treatment of advanced non-small cell lung cancer: where are we with evidence? Curr Surg Rep 2018;6:5. [Crossref] [PubMed]
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [Crossref] [PubMed]
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: 7-year follow-up of cancer and leukaemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210-5. [Crossref] [PubMed]
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Reymen B, van Baardwijk A, Wanders R, et al. Long-term survival of stage T4N0-1 and single station IIIA-N2 NSCLC patients treated with definitive chemoradiotherapy using individualised isotoxic accelareted radiotherapy (INDAR). Radiother Oncol 2014;110:482-7. [Crossref] [PubMed]
- Ahn JS, Ahn YC, Kim JH, et al. Multinational radomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KC-SG-LU05-04. J Clin Oncol 2015;33:2660-6. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Sculier JP, Lafitte JJ, Berghmans T, et al. A phase III randomised study comparing concomitant radiochemotherapy with cisplatin and docetaxel as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2018;117:32-7. [Crossref] [PubMed]
- Dempke WC, Sellmann L, Fenchel K. Immunotherapies for NSCLC: are we cutting the Gordian Helix? Anticancer Res 2015;35:5745-57. [PubMed]
- Dempke WC, Fenchel K, Uciechowski P, et al. Second- and third-generation drugs for immuno-oncology treatment – the more the better? Eur J Cancer 2017;74:55-72. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Mezquita L, Planchard D. Durvalumab in non-small-cell lung cancer patients: current developments. Future Oncol 2018;14:205-22. [Crossref] [PubMed]
- Garassino MC, Vansteenkiste JF, Kim J, et al. Durvalumab in ≥ 3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. 2016 World Conference on Lung Cancer. Abstract PL04a.03.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Rizvi NA, Peters S. Immunotherapy for unresectable stage III non-small-cell lung cancer. N Engl J Med 2017;377:1986-8. [Crossref] [PubMed]
- Sepesi B, Nelson DB, Mitchel KG, et al. Progonstic value of PD-L1 mRNA sequencing expression profile in non-small cell lung cancer. Ann Thorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Fiorica F, Belluomini L, Stefanelli A, et al. Immune checkpoint inhibitor nivolumab and radiotherapy in pretreated lung cancer patients: efficacy and safety of combination. Am J Clin Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Chen Y, Peng X, Zhou Y, et al. Comparing the benefits of chemoradiotherapy and chemotherapy for resectable stage IIIA/N2 non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2018;16:8. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Eberhardt W, Gauler T, Pöttgen C, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA (N2) and selected IIIB non-small cell lung cancer (NSCLC) after induction chemotherapy and concurrent CRTx (ESPATUTE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]


