Osimertinib in untreated epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer
Lung cancer remains the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% of all cases of lung cancer and is commonly diagnosed at an advanced stage of disease. For a long time, platinum-based chemotherapy has represented the cornerstone for the first-line treatment of advanced NSCLC patients (2), although with several limitations, including a number of side effects and a dismal overall survival (OS). The identification of activating mutations in the tyrosine-kinase (TK) domain of the epidermal growth factor receptor (EGFR) gene and the development of specific targeted agents, namely tyrosine kinase inhibitors (TKIs), has marked the advent of the era of precision medicine, which has revolutionized the diagnostic and therapeutic approach to NSCLC. Beyond EGFR-TKIs, thanks to an improved knowledge of the biology of lung tumors, during the last few years, the treatment landscape has been further expanded with the development of additional oncogene-directed agents, including anaplastic lymphoma kinase (ALK)- and BRAF-inhibitors (3). These therapies have significantly improved survival and quality of life in molecularly defined subgroups of NSCLC patients.
Gefitinib, erlotinib and afatinib represent the standard first-line treatment for advanced EGFR mutated NSCLC patients. Gefitinib and erlotinib are 4-anilinoquinazoline derivatives (4), which act as competitive reversible inhibitors by preventing the binding between the adenosine triphosphate (ATP) and the TK domain of the EGFR. Afatinib, structurally similar to gefitinib and erlotinib, is a dual EGFR/HER2 TKI, that irreversibly alkylates the cysteine 797 in the ATP binding site (5). Results from randomised phase III trials comparing the efficacy of these EGFR-TKIs with platinum doublet chemotherapy in EGFR-mutated NSCLC demonstrated a significantly higher objective response rate (ORR) and longer progression-free survival (PFS) in patients receiving EGFR-TKIs (6-8). Probably due to crossover effect, no OS difference was observed between EGFR-TKIs and chemotherapy across all phase III studies. The phase IIB Lux-Lung 7 was the first, prospective, randomised head-to-head trial, comparing the efficacy of an irreversible EGFR TKI, afatinib, with the reversible one gefitinib (9). Afatinib resulted in significantly longer PFS, time to treatment failure (TTF) and higher ORR, even though no OS difference was observed between the two arms. Despite initial remarkable responses, after a variable length of time from starting treatment, generally within 1 year, secondary resistance mechanisms to first- and second-generation EGFR-TKIs inevitably emerge, and patients develop disease progression. Many efforts have been made to define the mechanisms of acquired resistance to EGFR-TKIs by rebiopsying tumor specimens at progression. Different mechanisms have been identified and can be broadly categorized into the following types: secondary mutations within the EGFR kinase domain, activation of alternative signaling pathways, and phenotypic changes, including small cell lung cancer transformation and epithelial to mesenchymal transition (10). A secondary mutation at exon 20 within the kinase domain of EGFR, the T790M mutation, is the most common mechanism of acquired resistance, occurring in approximately 50–60% of EGFR-TKI-resistant tumors. This gatekeeper mutation interferes with EGFR-TKIs binding through a steric hindrance, restoring the affinity of mutant receptor for ATP, and reducing the potency of competitive inhibitors. Several treatment strategies have been developed to overcome resistance in EGFR T790M mutation-positive NSCLC. Third-generation, mutant EGFR-selective TKIs have been designed to preferentially target sensitizing mutations, as well as the T790M resistance mutation, over the wild-type receptor, thus, likely reducing on-target toxicities. Among these, osimertinib (11) has been the first compound granted US Food and Drug Administration (FDA) and European Medicine Agency (EMA) approval for the treatment of patients with metastatic EGFR T790M-positive NSCLC, progressing to prior EGFR-TKI therapy (12).
The randomized, phase III AURA3 study showed the superiority of osimertinib in terms of PFS and ORR with a better safety profile over platinum-pemetrexed chemotherapy in T790M-positive advanced NSCLC patients, who had progressed to first-line EGFR-TKIs, thus establishing osimertinib as the standard of care in this setting (13). Results from two expansion cohorts of the phase I AURA trial suggested osimertinib might be effective as up-front therapy in EGFR-mutated, advanced NSCLC patients (14). Osimertinib was associated with a high ORR of 77% (67% and 87% in the cohort of patients receiving 80 and 160 mg once daily, respectively), and a disease control rate (DCR) of 93% and 100% in the 80- and 160-mg group, respectively. Median PFS was 22.1 months in the 80-mg group, 19.3 months in the 160-mg group, and 20.5 months across both doses.
Based on promising efficacy and tolerability data from the AURA first-line cohorts, the 80-mg dose was selected for further investigation in the phase III, randomized FLAURA trial, designed to demonstrate PFS improvement of osimertinib over first-generation EGFR-TKIs in EGFR-mutated naïve NSCLC patients (15). Results from this study have been recently published. A total of 156 NSCLC patients, stratified according to the type of EGFR mutation (exon 19 deletion or L858R) and race, were randomized 1:1 to receive osimertinib 80 mg or a first-generation EGFR-TKI (gefitinib 250 mg or erlotinib 150 mg once daily). The primary endpoint was PFS, as assessed by investigators, according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Treatment beyond disease progression was allowed at investigators judgement in case of clinical benefit. Cross-over to osimertinib was allowed at disease progression in patients receiving standard EGFR-TKI after documentation of T790M-positive mutation status on plasma or tissue. Osimertinib significantly prolonged PFS over standard EGFR-TKI [18.9 versus 10.2 months; hazard ratio (HR) for disease progression or death, 0.46; 95% CI, 0.37–0.57; P<0.001], representing a 54% lower risk of disease progression or death. The benefit was observed across all predefined subgroups of patients, including those with central nervous system (CNS) metastases (15). A blinded independent central review confirmed PFS results from investigator assessment. Although there were no significant differences in ORR between the two arms, osimertinib was associated with higher DCR (97% versus 92%; odds ratio, 2.78; 95% CI, 1.25–6.78; P=0.01), depth of response and a longer duration of response (DoR: 17.2 versus 8.5 months) over gefitinib or erlotinib. At the time of data cutoff, only 25% of the events had occurred, and the OS benefit for osimertinib did not reach statistical significance. A cross-over effect could not be excluded, since approximately half of the patients in the standard EGFR-TKI arm with documented T790M-positive EGFR mutations at the time of tumor progression crossed to osimertinib. Notably, a lower rate of grade ≥3 adverse events (AEs) and of AEs leading to treatment discontinuation was reported in patients receiving osimertinib, despite the longer time of exposure to this drug, suggesting a better tolerability profile compared to standard EGFR-TKIs.
These robust data in terms of efficacy and safety of osimertinib have opened new questions regarding its use as up-front therapy in EGFR-mutated NSCLC patients, or at time of disease progression following treatment with first- or second-generation EGFR-TKIs. A biologic interpretation of the results from FLAURA is that the greater benefit of up-front osimertinib and the early separation of the Kaplan-Meier curves, observed at the first radiological evaluation, could indicate the presence of baseline EGFR T790M mutation in a subpopulation of patients. The presence of EGFR T790M in pretreatment tumors has been reported at different frequencies, depending on the detection methods used. Indeed, the T790M mutation, assessed by using a highly sensitive method based on peptide nucleic acid clamping PCR, was observed in approximately 65% of baseline tumor samples of patients enrolled in the EURTAC study (16) and was associated with shorter PFS (15.8 versus 9.7 months in patients without or with T790M, respectively), suggesting that it might interfere and reduce the benefit from standard EGFR-TKIs. In this context, up-front treatment with osimertinib might inhibit at an early stage or prevent T790M-mediated resistance. The ongoing phase II AZENT study (NCT02841579), that is evaluating the efficacy of osimertinib in terms of ORR in NSCLC patients carrying EGFR activating mutations and the EGFR T790M mutation at diagnosis, will address this issue.
The current approval of osimertinib underline the need of genotyping post-progression tumor samples for EGFR T790M, which is currently recommended by NSCLC guidelines for guiding treatment decisions. Tumor tissue rebiopsy at progression has several disadvantages in clinical practice, and it is not easily obtained, due to accessibility of metastatic sites, or insufficient materials (17). Tumor rebiopsy represents a single snapshot of the tumor that might not reflect its clonal heterogeneity, thus underscoring the presence of activated oncogenes in cellular subclones. Moreover, tumor tissue specimens cannot be easily obtained repeatedly to track tumor dynamics over time, because most diagnostic procedures are invasive and unacceptable to the patients. In recent years, the role of liquid biopsy for noninvasive assessment of tumor specific biomarkers, including EGFR mutations, has been widely established (17). Exploratory analyses from the AURA studies have demonstrated that T790M mutation assessment in circulating cell-free tumor DNA (ctDNA) can be a viable surrogate of tumor tissue profiling to predict response to osimertinib (12). Therefore, the current approval of osimertinib includes the possibility of determining EGFR T790M mutation status at time of progression to a prior EGFR-TKI therapy using either a validated plasma-based or tissue-based test. However, if the result of plasma detection in plasma is negative, due to the potential for false negative results, a tissue test is advisable wherever possible to determine EGFR T790M mutation (18). Data from literature indicate that digital droplet PCR (ddPCR), performed in ctDNA, identifies EGFR T790M mutation at a median time of approximately two months before RECIST progression (19). However, the biologic and clinical value of this finding is still unknown. With the aim to compare a sequence strategy (gefitinib followed by osimertinib) with osimertinib up-front and determine the role of liquid biopsy to define the timing of switching from gefitinib to osimertinib, the phase II APPLE trial (EORTC 1613) has been designed. A total of 156 EGFR mutated NSCLC patients will be randomized between osimertinib, or gefitinib followed by osimertinib at the time of the emergence of T790M in blood independent of RECIST progression, or gefitinib followed by osimertinib at the time of disease progression. The primary end point is PFS rate at 18 months. The activity of osimertinib to prevent the development of brain metastases will be also evaluated. Indeed, another major finding from the FLAURA trial, supporting the use of osimertinib up-front, regards its efficacy against CNS metastases. Despite a great amount of data reporting a certain activity of early generation EGFR-TKIs in EGFR-mutated NSCLC patients with brain and leptomeningeal metastases, approximately 30% of patients develop brain progression while receiving these agents, mainly due to their poor blood-brain barrier (BBB) penetration. Osimertinib is a substrate of the P-glycoprotein and BCRP efflux transporters. However, thanks to its passive permeability profile, studies in rat and monkeys indicate that it can achieve a faster and greater brain distribution compared to other EGFR-TKIs, including gefitinib, afatinib and rociletinib (20). The CNS activity of osimertinib has been recently demonstrated in a pooled analysis including data from patients enrolled in the two phase II studies, the AURA extension and the AURA2 (21). A total of 194 patients out of 411 enrolled had brain metastases at the time of study enrollment. Of these, 74% had previously received brain radiotherapy. The CNS ORR observed was of 54%, with a CNS DCR of 92%. These results were prospectively confirmed in the phase III AURA3 study, including also patients with stable, asymptomatic CNS metastases (13). CNS assessment was performed by blinded independent central review and in the subgroup of patients with measurable disease, the CNS ORR was significantly higher in patients receiving osimertinib compared with chemotherapy (70% versus 31%; P=0.015) and responses were more durable. For the 116 patients with baseline measurable and non-measurable CNS disease, median PFS was 11.7 months with osimertinib and 5.6 months with chemotherapy (HR, 0.32, P=0.004). Notably, the probability of CNS progression was lower than for chemotherapy at 3 and 6 months. The FLAURA study further confirmed a superior CNS efficacy of osimertinib compared to standard EGFR-TKIs, with a PFS benefit similar to that of patients without brain metastases (15). In the AURA3, osimertinib showed activity also against leptomeningeal metastases. This activity has been also supported by preliminary results from the phase I BLOOM study, specifically investigating the safety of osimertinib at 160 mg daily in patients with leptomeningeal disease, confirmed by positive cerebrospinal fluid (CSF) (22). Overall, these data suggest osimertinib can be a valid therapeutic option for both patients with and without brain metastases. This could represent a significant challenge, especially considering that brain radiation might decrease cognitive function, reduce memory and compromise the quality of life in a subgroup of metastatic patients, who have a high probability of extended survival.
Currently, we do not have data regarding the activity of osimertinib in patients harboring rare EGFR mutations. There are few reports about the efficacy of standard EGFR-TKIs on these mutations with discordant results. The largest prospective data come from a pooled analysis, including patients enrolled in the Lux-Lung2, Lux-Lung3 and Lux-Lung6 trials, in which 75 NSCLC patients with uncommon EGFR mutations received afatinib (23). Among these, 14 harbored de novo T790M alone or in combination with exon 19 deletions or exon 21 mutations, 23 carried exon 20 insertions and 38 had other rare mutations in exons 18, 19, 20 and 21. Approximately 70% of the 38 patients carrying exon 18 (G719X), 20 (S768I) and 21 (L861Q) mutations had an objective response, with a median DoR of 11 months and a DCR of 84%. Additional studies are warranted to investigate the role of osimertinib in this subgroup of patients.
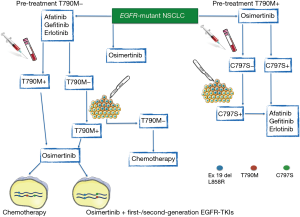
An emerging issue regards the therapeutic strategy after progression to osimertinib. Heterogeneous molecular mechanisms of acquired resistance have been described, including the onset of the secondary EGFR C797S mutation (24), that might occur in cis or in trans with the T790M. While the presence of C797S and T790M in cis determines resistance to all three generations of EGFR-TKIs, a combination of first and third-generation inhibitors would be effective in case of C797S and T790M mutations in trans, as suggested by some reports (24) (Figure 1). However, we have to consider that the available data derive from liquid biopsies performed in patients treated with both standard EGFR-TKIs and osimertinib. We do not know the prevalence of C797S and other resistance mechanisms after osimertinib front-line. Patients could develop C797S in the absence of T790M mutation, and standard EGFR-TKIs, that do not depend on the cysteine at position 797, could be a valid second-line therapy in this setting (Figure 1).
Albeit the impressive efficacy of osimertinib in naïve EGFR-mutated NSCLC patients, the activation of compensatory signaling pathways, including SRC family kinases (SFKs), focal adhesion kinase (FAK) and the up-regulation of receptor tyrosine kinases (RTKs) might occur, thus contributing to osimertinib resistance (25). In vitro and in vivo studies indicate that a combinatorial strategy, including osimertinib with the Src/FAK/JAK2 inhibitor TPX-0005 efficiently inhibits the downstream signaling, thus providing preclinical evidence that osimertinib monotherapy might not be adequate. Molecular profiling of tumor from tissue rebiopsies or from ctDNA collected at different time points during first-line treatment with osimertinib and at progression will be crucial to elucidate resistance mechanisms and suggest effective therapeutic strategies.
In conclusion, based on results from FLAURA study, osimertinib represents a new therapeutic opportunity for EGFR-mutated, TKI-naïve NSCLC patients. Based on the above commented efficacy and safety data from this large phase III trial, when the regulatory process for its approval will be completed, it could be considered as a therapeutic option preferable to early generation EGFR-TKI, especially in those patients with CNS involvement.
Based on its favorable toxicity profile, osimertinib is considered a particularly attractive candidate for combinatorial approaches that may potentially further improve the clinical outcome of EGFR-mutated patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Santarpia M, Karachaliou N, Rosell R. Beyond platinum treatment for NSCLC: what does the future hold? Expert Rev Anticancer Ther 2017;17:293-5. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Leveque D. Pharmacokinetics of gefitinib and erlotinib. Lancet Oncol 2011;12:1093. [Crossref] [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Santarpia M, Gil N, Rosell R. Strategies to overcome resistance to tyrosine kinase inhibitors in non-small-cell lung cancer. Expert Rev Clin Pharmacol 2015;8:461-77. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Santarpia M, Liguori A, Karachaliou N, et al. Osimertinib in the treatment of non-small-cell lung cancer: design, development and place in therapy. Lung Cancer (Auckl) 2017;8:109-25. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]
- Santarpia M, Karachaliou N, Gonzalez-Cao M, et al. Feasibility of cell-free circulating tumor DNA testing for lung cancer. Biomark Med 2016;10:417-30. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep 2016;6:20913. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two Phase II trials. Ann Oncol 2018;29:687-93. [Crossref] [PubMed]
- Yang JC, Kim DW, Kim SW, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. J Clin Oncol 2016;34:9002.
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Karachaliou N, Chaib I, Cardona AF, et al. Common Co-activation of AXL and CDCP1 in EGFR-mutation-positive Non-smallcell Lung Cancer Associated With Poor Prognosis. EBioMedicine 2018;29:112-27. [Crossref] [PubMed]


