Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience
Introduction
Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancers divided into non-squamous (70%) and squamous (30%) histologic subtypes. Metastatic disease is present in 50% of new NSCLC diagnoses and the prognosis for these patients with metastatic or stage IV NSCLC is extremely poor with 5-year survival rates reported as less than 5% (1).
Till now, for metastatic wild type NSCLC (80%), the standard first-line treatment has been platinum-based doublet chemotherapy with response rates (ORR) ranging only between 15–30% and overall survival (OS) of 8–10 months (2). For those patients with non-squamous carcinoma, this chemotherapy might be administered alone or in combination with bevacizumab (3,4), for four-to-six cycles, followed by maintenance treatment with pemetrexed or bevacizumab (5). With the detection of activating epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) or ROS1 translocation/rearrangements the targeted agents improved the ORR to 70% and OS to more than 20 months, but few patients harbor these alterations (20%) and resistance to treatment frequently occurs (6,7).
During the last decade, the only approved agents for the second line treatment for patients without molecular target aberrations were two cytotoxic agents, docetaxel and pemetrexed (last one only for non-squamous histology) (8-10) and the tyrosine kinase inhibitor (TKI) erlotinib (11). Survival benefit with the second line treatments is modest with response rates (RR) 5–10%, median progression-free survival (PFS) of 2–4 months, and median OS of 6–9 months. Thus, there was an unmet need for the second line treatment options capable to improve efficacy.
In the last few years, the development of immune checkpoint inhibitors has totally changed the therapeutic landscape of NSCLC and modified the standards of treatments. It is the first time that a significant percentage of patients with the diagnosis of metastatic NSCLC are still alive from the beginning of the treatment in comparison with historical reports of chemotherapy.
Nivolumab (12,13) and pembrolizumab (14), two immune checkpoint inhibitors targeting programmed cell death-1 (PD-1), improved the OS in different phase III trials in comparison with docetaxel and they were approved in 2015 for second-line therapy of NSCLC. Another monoclonal antibody designed to bind with programmed death-ligand 1 (PD-L1), atezolizumab (15), demonstrated again in 2016, betters results than docetaxel and it was approved for the same indication. Moreover, pembrolizumab was compared with a platin combination in first line of NSCLC and in patients with high PD-L1 expressing tumors was superior getting a median SG of 30 months (16) (Table 1).
Full table
In this way, nivolumab, was the first checkpoint inhibitor to show a survival benefit in previously treated patients with advanced squamous and non-squamous NSCLC in two randomized trials in comparison with docetaxel; the CheckMate 017 and CheckMate 057 (11,12). It is a fully human IgG4 PD-1 immune-checkpoint-inhibitor antibody, which disrupts PD1-mediated signaling and restores antitumor immunity.
In addition to efficacy it is necessary to understand and manage the toxicity profile of immunotherapy agents compared with chemotherapy. Inhibition of the immune checkpoints produce an immune dysregulation activating the T cells and in this way they can lead to autoinmune diseases. These side effects include dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary events and symptoms and they are known as immune-related adverse events (AEs). The PD-1/PD-L1 inhibitors can also produce the classical chemotherapy toxicities such as fatigue, anorexia, nausea, and diarrhea (17).
Experience in routine clinical practice may differ from that seen in a controlled clinical trial. This is an observational study that represents the real world experience in Galician previously treated NSCLC. The main aim of this study is to analyze the characteristics, the treatment outcomes and safety of patients with advanced stage NSCLC treated with nivolumab in second line in our clinical practice and correlate the results with the evidence from the clinical trials. Moreover, a secondary aim is to perform subgroup analysis in order to identify differences in survival outcomes by clinical features.
Methods
Patients
This study was a multicenter, retrospective and observational systematic review. Retrospectively, there were collected clinical records from advanced NSCLC patients treated with nivolumab from August of 2015 to January of 2017 in 9 different Galician centers and a total of 188 patients were enrolled.
The treatment plan was explained in detail, informed consent was obtained from all patients and the study was approved by the Galician Local Research Ethics Committees (GGC-NIV-2018-01) nd was executed in accordance with the Declaration of Helsinki, Good Clinical Practice, and local ethical and legal requirements.
Eligibility criteria included, histologically or cytologically confirmed NSCLC clinical stage IIIB and IV, evaluable disease, at least one prior therapy, a performance status of 0, 1 or 2 and an adequate organ function. Exclusion criteria included, positive test for hepatitis B or C virus indicating acute or chronic infection, known history of testing positive for human immunodeficiency virus (HIV), a severe autoimmune disease and patients with a condition requiring systemic treatment with either corticosteroids (>10 mg daily prednisone equivalent) or other immunosuppressive medications.
The evaluation included a review of demographic data and tumour characteristics: age, gender, smoking status, performance status, clinical stage, central nervous system (CNS) metastases, number and duration of the previous treatments and presence of driver mutations as EGFR, ALK or ROS1.
Study design
Patients with advanced NSCLC after progression to one or more lines of chemotherapy received nivolumab 3 mg/kg IV (60 min) every 2 weeks either until progressive disease (PD) or unacceptable toxicity and computed tomography (CT) based evaluation was performed around every 12 weeks.
End points and assessments
As we said, the main aim of the study is to report the characteristics of these patients and the treatment outcomes and safety. Secondary aim of the study is to identify differences in survival outcomes by performing subgroup analysis.
The exploratory assessments include RR, PFS and OS. The PFS was calculated from the first day of administration of nivolumab until any relapse, local or distant, or death (whichever occurred first) and it was scored based on available clinical or imaging reports of any progression (local or distant). The OS, was calculated also from the first day of administration of nivolumab treatment until the last date of follow-up or death of the patient.
Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Safety was measured using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, and for specific immune-related AEs, there were used the protocol specific treatment guidelines.
Statistical analysis
A descriptive statistical analysis was performed, including central tendency and dispersion parameters for the quantitative variables and absolute and relative frequencies for categorical variables. Categorical variables were presented by numbers and percentiles, medians and ranges were reported for continuous variables.
OS for categorical variables and PFS were assessed by Kaplan-Meier method, with the log-rank test. OS for continuous variables was assessed by the Cox proportional-hazards regression model. Two-sided P values less than 0.05 were considered statistically significant.
Demographic analysis included all patients who underwent randomization (intention-to-treat population). Efficacy analysis included all patients evaluable and safety analysis included all the treated patients (those who received at least one dose of study drug).
Results
Patients’ characteristics
From August of 2015 to January of 2017, overall 188 patients were enrolled in the study from 9 different Galician centers.
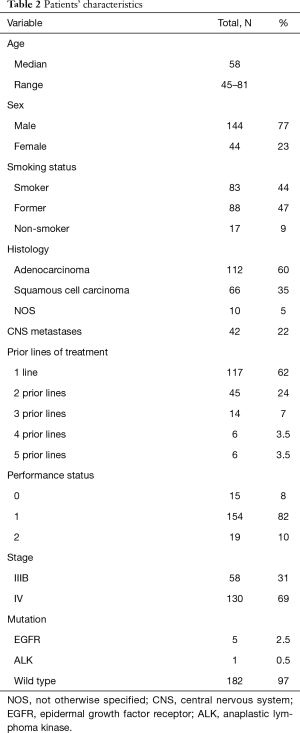
Baseline demographics and clinical characteristics are displayed in Table 2. The median age was 58 years and the majority of the patients were males 144 (77%) and former/current smokers 171 (91%). Patients with non-squamous histology predominated; 112 (60%) adenocarcinoma, 66 (35%) squamous-cell carcinoma and 10 (5%) NOS (not otherwise specified).
Full table
It should be mentioned that 39 (7.5%) patients were ≥70 years old, 42 (22%) of the patients had CNS metastases and 19 (10%) patients had PS 2, knowing that these three characteristics are usually under-represented in clinical trials.
All patients have previously received at least one platinum-based therapy and 71 (38%) patients received 2 or more prior systemic therapy lines; 45 (24%) two lines, 14 (7%) three lines, 6 (3.5%), four lines and other 6 patients (3.5%) six lines. So we had a cohort with a high percentage of multi-treated patients.
The majority of patients (97%) included in the analysis did not harbor any molecular abnormality; only 5 patients were EGFR positive (2.5%) and 1 patient ALK positive (0.5%). All of them received first targeted therapy with TKI followed by platinum doublet and finally followed by nivolumab.
In our cohort, no routine tumor staining for PD-L1 was done because in that moment it wasn’t something necessary in the routine clinical practice.
Efficacy: RR, PFS and OS
All patients included in the analysis received at least one cycle of nivolumab at the standard dose of 3 mg/kg every 2 weeks. The median number of cycles administered was 6 (range, 1–34).
Forty-three patients (22.8%) had required at least one dose delay because of toxicity.
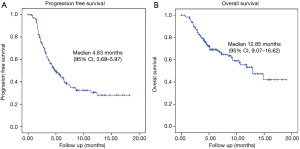
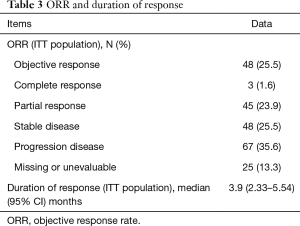
Of the 188 patient enrolled at the data cut off, 64 (34%) have died and 25 (13.3%) patients were not evaluated because no data were available. Among 163 patients evaluated, 3 (1.6%) had complete response, 45 (23.9%) partial response, 48 (25.5%) stable disease and 67 (35.6%) progression disease (Table 3). At the time of database analysis, the median of PFS was 4.83 months (95% CI, 3.69–5.97) and OS was 12.85 months (95% CI, 9.07–16.62) (Figure 1). The median duration of response was 3.9 months (95% CI, 2.33–5.54) and the OS at 6 months is 70%, at 12 months 55% and at 18 months 42%.
Full table
Safety
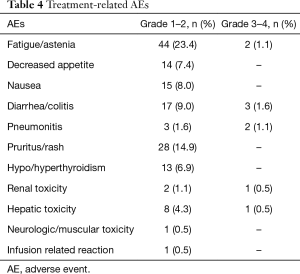
Treatment-related AEs occurred in 78% of the patients and are listed in Table 4. Generally side effects (76.6%) were grade 1–2 and only in 9 (4.8%) patients were reported grade 3–4.
Full table
The most common adverse effects were fatigue/asthenia 44 (23.4%), nausea 15 (8%) and decreased appetite 14 (7.4%). Two patients presented asthenia grade 3 and it was necessary to stop the treatment.
Among the specific immune-related AEs instead, skin toxicity, like rash and pruritus, was the most frequent one which appeared in 28 (14.9%) patients. In all of them the symptoms disappeared with topic corticosteroids.
The second most frequent specific immune-related AE was the diarrhea. Seventeen (9%) patients presented grade 2 diarrhea and they overcame with anti-diarrheal diet and pills but 3 patients developed grade 3 diarrhea and required hospitalization; one of them recovered with endovenous corticosteroids and liquids but the other two got worsen and complicated with a colitis and died because of an intestinal perforation.
There were also seen 13 (6.9%) patients with asymptomatic endocrinopathies; 12 of them presented hypothyroidism and the other 1 hyperthyroidism being controlled with pharmacological treatment and continuing treatment with nivolumab.
Nine (4.8%) cases of hepatitis with elevation of transaminases were seen; 8 (4.3%) patients presented grade 2 and asymptomatic and 1 (0.5%) of them grade 3 but with favorable evolution with oral corticosteroids.
Three (1.6%) patients developed grade 2 pneumonitis and resolved the symptomatology with oral corticosteroids. Two patients (1.1%) instead complicated with severe pneumonitis and after a long hospitalization with endovenous high doses of corticosteroids and multiple antibiotics, they finally recovered.
Three more patients presented renal toxicity; 2 patients (1.1%) with grade 2, but it was possible to restart the treatment with no new complications and one patient (0.5%) with grade 4 stop indefinitely the treatment.
There was an infusional reaction grade 1 but didn´t recur with subsequent dose after appropriate premedication and there was a rare case of peripheral neuropathy grade 2.
In summary, grade 3–4 toxicities were reported in 9 (4.8%) patients (3 diarrhea, 2 pneumonitis, 2 asthenia, 1 hepatitis, 1 renal toxicity) and there were 2 (1%) treatment-related deaths, both because of an intestinal perforation.
Discontinuation of the study drug due to treatment-related AEs occurred in 9 (4.8%) patients in total and 38 (20.2%) patients required at least one dose delay of the treatment.
Subgroup analysis
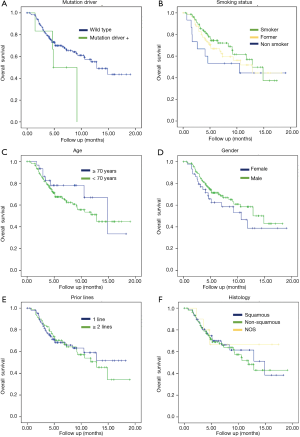
Subgroup analyses were performed for OS. There were not statistically significant differences regarding gender (female 10.6 months vs. male 14.8 months, P=0.23), age (patients <70 years 12.8 months vs. patients ≥70 years 14.85 months, P=0.32), histology (non-squamous 11.7 vs. 14.8 months for squamous, P=0.74) or number of prior therapy lines (1 prior line NR vs. 2 or more prior lines 12.8 months, P=0.76).
It was a trend toward improved OS but no significant correlation in the next two parameters: tobacco status (current smokers lived 12.8 months vs. former smokers 12.8 months vs. never smokers 10.5 months, P=0.4 respectively) and mutation status (EGFR/ALK wildtype 12.8 vs. 4.8 months in patients with mutation positive, P=0.12) (Figure 2).
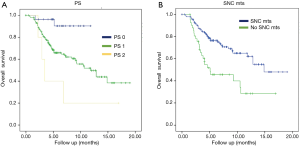
The subgroup analysis revealed statistical significance in OS for patients without SNC metastases vs. patients presenting them with the results of 14.8 months (95% CI, 11.5–17.3) vs. 5.09 months (95% CI, 0.3–9.8) with P=0.0001, respectively. Another statistically significant positive factor in the univariate analysis was PS: patients with PS 2 had an OS of 3.4 months (95% CI, 2.3–4.4) vs. patients with PS 1 that the OS was 11.79 months (95% CI, 8.5–15) and finally, patients with PS 0 the OS wasn’t reached, with P=0.006 (Figure 3, Table 5).
Full table
Discussion
Immunotherapy is nowadays considered the new revolution in cancer therapy and also in NSCLC. PD-1 inhibitors, nivolumab and pembrolizumab, and PD-L1 inhibitor, atezolizumab, have become the new standard of care for second-line treatment of NSCLC and in addition, pembrolizumab has also been approved for the first-line treatment of patients with metastatic NSCLC whose tumors have ≥50% of PD-L1 expression.
There is not a perfect biomarker but the majority of the studies have demonstrated that there are better responses when the expression of PD-L1 on tumor cells is higher. It is also known that some patients with no or low levels of PD-L1 expression have good responses to these agents so it is necessary to continue investigating to determine which ones are the best response predictive factors.
Real world data (RWD) on this therapy is scarce and it is important to evaluate the translation of the benefit shown in the clinical trials to a broader and unselected lung cancer population of the real world setting (18-21). For the best of our knowledge, this study represents probably one of the biggest real-word experience published with nivolumab in advanced NSCLC (both histologies) after progression to one or more lines of chemotherapy. The results are consistent with those previously reported in published data from phase III trials (12,13,22).
In nivolumab clinical program development, both NSCLC histologies (squamous and non-squamous) were analyzed independently in two separate clinical trials where population was enrolled without PDL-1 selection.
In the CheckMate 017, squamous NSCLC patients were involved and were observed higher RRs (20% vs. 9%), longer duration of response (25.2 vs. 8.4 months) and median OS (OS 9.2 vs. 6.0 months; HR: 0.62, 95% CI, 0.47–0.80) in nivolumab treated patients versus docetaxel. The median PFS was 3.5 months with nivolumab versus 2.8 months with docetaxel (hazard ratio for death or disease progression, 0.62; 95% CI, 0.47–0.81; P<0.001) and the PFS rate at 1 year was 21% vs. 6% (nivolumab vs. docetaxel). PD-L1 expression stratified to 1%, 5%, and 10% was neither prognostic nor predictive of benefit (12,22).
On the other hand, the non-squamous population was studied in the CheckMate 057 trial. Again higher RRs (19% vs. 12%), longer duration of response (17 vs. 5.6 months) and median OS (OS 12.2 vs. 9.5 months; HR: 0.75, 95% CI, 0.63–0.91) was also observed in nivolumab arm vs. docetaxel. Nivolumab was associated with even greater efficacy than docetaxel across all subgroups defined according to tumor-membrane expression of PD-L1 (≥1%, ≥5%, and ≥10%). Although the median progression free survival did not favor nivolumab over docetaxel (2.3 and 4.2 months, respectively), the rate of progression free survival at 1 year was higher with nivolumab than with docetaxel (19% and 8%, respectively) (13,22).
In our study, where both histologies were included, the RR observed was 24.6%; 45 (23%) patients had partial response and 3 (1.6%) patients had complete response (95% CI, 31.73–19.27), the median of PFS was 4.83 months (95% CI, 3.6–5.9) and OS was 12.8 months (95% CI, 9.07–16.62). The median duration of response was 3.9 months (95% CI, 2.33–5.54) and the OS at 6 months was 70%, at 12 months 55% and at 18 months 42%.
In the subgroup analysis, there were no statistical significance in gender, age, histology and prior therapy lines. However, it was a trend toward improved OS in the next two parameters: tobacco status (current smokers lived 12.8 months vs. former smokers 12.8 months vs. never smokers 10.5 months, P=0.43 respectively) and mutation status (EGFR/ALK wildtype 12.8 vs. 4.8 months in patients with mutation positive, P=0.12).
This finding is similar to results reported in published data in this clinical setting and the hypothesis is that PD-1 and PDL-1 inhibitors have less activity in never smokers and in patients EGFR/ALK mutation positive because these patients might have low mutational heterogeneity and immunogenicity (23). In the CheckMate 057 trial, the hazard ratios in the analysis of OS favored nivolumab across most pre-specified patient subgroups; the exceptions were who had never smoked and those with EGFR mutation-positive status (13). In RWD, Garassino et al. presented an analysis of patients with advanced non-squamous never smokers and EGFR positive tumours. Median OS was 10.0 months (95% CI, 8.1–11.9) for never-smokers, 8.3 months (95% CI, 2.2–14.4) for patients with an EGFR-positive tumor, 5.6 months (95% CI, 3.4–7.8) for never-smokers with an EGFR-positive tumor, and 11.0 months (95% CI, 10.0–12.0) for all patients. They concluded that Nivolumab should be considered as a therapeutic option for previously treated patients with advanced NSCLC whose tumors harbor EGFR mutations, despite apparently lower OS rates compared with the overall cohort (24).
On the other hand, in our study, the analysis revealed statistical significance in OS for patients without CNS vs. patients presenting them with the results of 14.8 months (95% CI, 11.5–17.3) vs. 5.09 months (95% CI, 0.3–9.8) with P=0.0001, respectively. Another statistically significant positive factor in the univariate analysis was PS: patients with PS 2 had an OS of 3.4 months (95% CI, 2.3–4.4) vs. patients with PS 1 that the OS was 11.79 months (95% CI, 8.5–15.07) and finally, patients with PS 0 the OS wasn’t reached, with P=0.006. These expected results could be explained because patients with CNS metastases and PS 2 usually have worse prognosis than ones without CNS metastases and PS 0 or 1.
In the clinical development program of nivolumab, patients with pretreated CNS metastases were permitted and a pool analysis in this population was presented. This analysis revealed a similar safety profile to that previously reported in the overall population, no additional treatment-related neurologic toxicities (e.g., cerebral edema) were observed and nivolumab resulted in longer OS than docetaxel 8.4 vs. 6.2 months (95% CI, 4.99–11.6) (25).
Regarding RWD in this setting, Bidoli et al. presented at ESMO 2016 an analysis in patients with advanced squamous NSCLC and CNS metastases from the expanded access program in Italy (26). The median OS was 5.8 months (95% CI, 1.8–9.8) for patients with CNS metastases and 7.9 months (95% CI, 6.2–9.6) for all patients. The OS rate at 12 months was 35% for patients with CNS metastases and 39% for all patients. Moreover, Crino et al. presented the same analysis but in non-squamous and CNS metastases. In this one, median OS was 8.1 months (95% CI, 6.2–10.0) for patients with CNS metastases and 11.0 months (95% CI, 10.0–12.0) for all patients. The OS rate at 1 year was 43% for patients with CNS metastases and 48% for all patients (27).
In both analysis, they concluded that efficacy of nivolumab in patients with CNS metastases was similar to that observed in the overall cohort of Italian patients. Comparison with our study it is not possible taking into account that our study has both histologies together, squamous and non-squamous, with different prognosis profile, but the results appear to be aligned.
On the other hand, it has been already reported efficacy and safety of nivolumab in old patients and patients with PS 2 from the CheckMate 153 trial (28). Nivolumab was effective and well tolerated in PS 2 patients, with no new safety signals detected and a median OS of 3.9 months (95% CI, 3.1–6.3) and 1 year OS rate of 23% (95% CI, 16–32%). In the preliminary data of CheckMate 171, a study conducted to observe the efficacy and safety of nivolumab in patients ≥70 years and PS 2 were similar (29). In RWD, Elizabeth et al. concluded that patients with PS 2 are associated with poor prognosis than PS 0–1 with a median OS of 3.5 months (95% CI, 2.6–4.5) vs. 9.5 months (95% CI, 6.7–NR) (30).
Finally, subgroup analyses were not powered for formal efficacy comparisons and should be interpreted with caution.
Overall, nivolumab was well tolerated, with a favorable AE profile and observed AEs were consistent with those previously reported. The proportion of patients who experienced grade 3 or 4 AEs, treatment-related AEs, and those leading to discontinuation of study treatment was low. We had two treatment-related deaths which occurred at the beginning of our experience and therefore at the beginning of our treatment management learning curve. Early detection and properly intervention are crucial to mitigate toxicity.
The limitations of this observational study are principally that is retrospective, in an unselected population and limited follow up. Moreover, there is an absence of a central radiological assessment for all patients included in the cohort and the PD-L1 staining has not been done.
In conclusion, we have shown a clinically meaningful survival benefit in nivolumab treated patients for previously treated advanced NSCLC with a favorable safety profile. These clinically relevant data support the use of nivolumab as a new treatment option for patients with advanced NSCLC whose disease has progressed during or after platinum-based chemotherapy in routine practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The treatment plan was approved by the Galician Local Research Ethics Committees (GGC-NIV-2018-01) and was executed in accordance with the Declaration of Helsinki, Good Clinical Practice, and local ethical and legal requirements. Informed consent was obtained from all patients participated.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Cufer T, Ovcaricek T, O'Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 2013;49:1216-25. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. Erratum in: N Engl J Med 2007;356:318. [Crossref] [PubMed]
- Kader YA, Le Chevalier T, El-Nahas T, et al. Comparative study analyzing survival and safety of bevacizumab/carboplatin/paclitaxel and cisplatin/pemetrexed in chemotherapy-naïve patients with advanced non-squamous bronchogenic carcinoma not harboring EGFR mutation. Onco Targets Ther 2013;6:803-9. [PubMed]
- Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol 2012;138:332-46. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Weiss JM, Stinchcombe TE. Second-Line Therapy for Advanced NSCLC. Oncologist 2013;18:947-53. [Crossref] [PubMed]
- Taxotere (docetaxel) injection concentrate, intravenous infusion (IV). Sanofi-aventis. Revised May 2010. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf. Accessed July 8, 2013.
- Eli Lilly and Company Limited. Alimta (pemetrexed) summary of product characteristics. 2012.
- Roche Products Limited. Tarceva (erlotinib) summary of product characteristics. 2014.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Crino L, Bidoli P, Delmonte P, et al. Italian cohort of Nivolumab expanded access program: preliminary data from a real-world population, Poster 3067. ASCO 2016 Annual Meeting. Chicago, IL, USA, June 3–7, 2016.
- Brustugun OT, Sprauten M, Helland Å. Real-world data on nivolumab treatment of non-small cell lung cancer. Acta Oncol 2017;56:438-40. [Crossref] [PubMed]
- Girard N, Valette CA, Cadranel J, et al. IFTC-1502 CLINIVO: Real life experience with nivolumab in patients (pts) with advanced Non Small Cell Lung Cancer (NSCLC): efficacy and safety of nivolumab and post-nivolumab treatment in the French Expanded Access Program (EAP). Ann Oncol 2017;28:v460-96. [Crossref]
- Fujimoto D, Yoshioka H, Kataoka Y, et al. P2.07-024 Real-World Data of Nivolumab for Previously Treated Non-Small Cell Lung Cancer Patients in Japan: A Multicenter Retrospective Cohort Study. J Thorac Oncol 2017;12:S2424-5. [Crossref]
- Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol 2016;27:1215PD.
- Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017;6:e1356145. [Crossref] [PubMed]
- Garassino M, Cortesi E, Grossi F, et al. Italian Nivolumab Expanded Access Program (EAP) in Non-Squamous NSCLC Patients: Real-World Results in Never-Smokers and EGFR-Positive Patients. Ann Oncol 2017;28:v460-96. [Crossref]
- Goldman JW, Crino L, Vokes EE, et al. P2.36: Nivolumab (nivo) in Patients (pts) With Advanced (adv) NSCLC and Central Nervous System (CNS) Metastases (mets): Track: Immunotherapy. J Thorac Oncol 2016;11:S238-9. [Crossref] [PubMed]
- Bidoli P, Chiari R, Catino A, et al. Efficacy and Safety Data From Patients With Advanced Squamous NSCLC and CNS Metastases Participating in the Nivolumab Expanded Access Program in Italy. Ann Oncol 2016;27:416-54. [Crossref]
- Crino L, Bidoli P, Roila F, et al. Efficacy and Safety Data From Patients With Advanced Non-Squamous NSCLC and Brain Metastases From the Nivolumab Expanded Access Program (EAP) in Italy. Ann Oncol 2017;28:v460-96. [Crossref]
- Spiegel DR, Schwartzberg LS, Waterhouse DM, et al. Is Nivolumab Safe and Effective in Elderly and PS 2 Patients With NSCLC? Results of CheckMate 153. The International Association for the Study of Lung Cancer 17th World Conference on Lung Cancer. Vienna, Austria: December 4–7, 2016.
- Popat S, Ardizzoni A, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: Results of a European, single arm, phase 2 trial (CheckMate 171) including patients aged ≥70 years or with poor performance status. Ann Oncol 2017;28:v460-96. [Crossref]
- Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]