Programmed cell death ligand-1 (PD-L1) as a biomarker for non-small cell lung cancer (NSCLC) treatment—are we barking up the wrong tree?
Immunotherapy with monoclonal antibodies targeting programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) has become a standard of care treatment for patients with advanced or metastatic non-small cell lung cancer (NSCLC) in first and later treatment lines. Prolonged and durable responses are seen in approximately 10–20% of patients treated, however, the efficacy of these checkpoint inhibitors requires an optimal selection of eligible patients who will benefit most. Clearly, there is a huge clinical need to establish validated biomarkers which are suitable to predict immunotherapy outcome in NSCLC patients (1).
PD-L1 expression has been extensively evaluated as a predictive biomarker for immunotherapy in NSCLC patients and has shown some value for predicting response to immune checkpoint inhibitors in some studies, but not in others. The use of PD-L1 as a biomarker remains to be complicated by a number of factors including the variability in tissue collection timing, the antibody and methodology used for staining (including the definition of positivity and the non-standardised test design), the heterogeneity and dynamic of PD-L1 expression within different tumours, and the role of PD-L1 expression on tumour-infiltrating lymphocytes and other immune cells versus the malignant cell population. In addition, PD-L1 is regarded to be a biological continuum and therefore might be of limited value as a biomarker in this subset of patients (2).
Pembrolizumab (Keytruda®, Merck, USA: targeting PD-1) is approved (EMA, FDA) for first-line treatment of NSCLC patients with advanced or metastatic cancers (with PD-L1 expression ≥50% using the Dako 22C3 IHC assay), whereas for second-line treatment a PD-L1 expression of ≥1% is required (1). Both, nivolumab (Opdivo®, Bristol-Myers Squibb, USA: targeting PD-1) and atezolizumab (Tecentriq®, Roche, Switzerland: targeting PD-L1) are also approved (EMA, FDA) in the second-line setting, but PD-L1 screening is not mandatory, however, complementary PD-L1 diagnostics are approved for NSCLC (1). Durvalumab (Imfinzi®, AstraZeneca, UK: targeting PD-L1) and avelumab (Bavencio®, MerckSerono, Germany: targeting PD-L1) are currently evaluated in first- or second-line treatment studies for NSCLC and are not approved for NSCLC treatment yet. However, it should be noted that durvalumab has demonstrated a significant progression-free survival (PFS) benefit [16.8 versus 5.6 months, hazard ratio (HR) =0.52] as maintenance therapy in stage IIIA/B NSCLC patients following radio-chemotherapy and two cycles of platinum-based therapy (PACIFIC trial) (3). Interestingly, PFS benefit was independent of PD-L1 expression. Details of this relevant phase III study is given in Table 1.
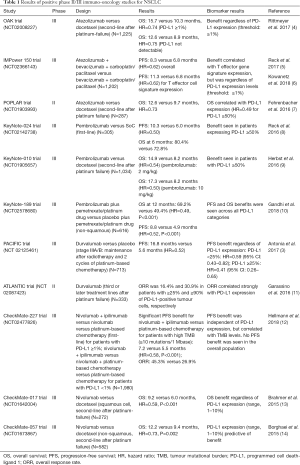
Full table
Some clinical trials (Table 1) have demonstrated that PD-L1 expression appears to be significantly correlated with clinical outcomes in NSCLC, but not in all trials, although some patients treated in these trials who had PD-L1-negative lung cancers, clinical benefit from PD-1/PD-L1 inhibitors was also observed. This observation adds therefore weight to the proposal that PD-L1 is a weak biomarker which prompted researchers to look beyond PD-L1 expression levels in order to identify more robust predictive biomarkers which then could help to better identify the immune status and the pre-existing tumour microenvironment of a given tumour.
To date, many groups of oncologists are attempting to establish better predictive biomarkers in NSCLC for monoclonal antibodies targeting the PD-1/PD-L1 axis to select patients who might have a greater benefit from immune checkpoint therapies. Initial preclinical and clinical studies have revealed that tumour mutational burden (TMB) or gene signatures may be an ideal strategy guiding treatment decisions for checkpoint inhibitors (15,16). Yarchoan et al. (15) reported a clear correlation between TMB and overall response rate (ORR) (P<0.001) following checkpoint inhibitor treatment in many cancers suggesting that a significant relationship between TMB and anti-PD-1/anti-PD-L1 treatment exists. On the other hand, Leal and Ramalingham (1) found that in NSCLC patients responding to immune checkpoint inhibitor treatment early PD-L1-positive CD8-positive T cell responses were seen suggesting that these proliferating CD8-positive T cells may have an effector-like phenotype which could generate cytotoxicity [see also (16) for a review].
Most recently, two phase III studies (CheckMate-227 and IMPower 150) have provided evidence that TMB correlates with the clinical response to the combination of nivolumab and ipilimumab (Yervoy®, Bristol-Myers Squibb, USA: targeting CTLA-4) (CheckMate-227) (12), whereas atezolizumab response was correlated with T effector gene signature expression (IMPower 150) (5,6,16). Both studies were conducted as first-line trials in NSCLC (Table 1). The CheckMate-227 trial is the first to our knowledge to evaluate TMB as a predictive biomarker for immunotherapy in NSCLC (co-primary endpoint). The study demonstrated a significant PFS benefit in the nivolumab + ipilimumab group in first-line NSCLC patients with a TMB level of ≥10 mutation/Mbase regardless of PD-L1 expression (7.2 versus 5.5 months, HR=0.58, P<0.001) (12). Interestingly, the reported median PFS benefit was only seen in the TMB subgroup, but not the overall population (12) suggesting that TMB may be a predictive novel biomarker for checkpoint inhibitor treatment. Objective response rates were found to be 45.3% versus 26.9%, respectively (12).
The IMPower 150 trial is the first phase III study to demonstrate a clinically meaningful and significant PFS benefit with atezolizumab plus bevacizumab and chemotherapy (paclitaxel and carboplatin) versus bevacizumab plus chemotherapy in the first-line stetting of advanced or metastatic NSCLC (8.3 versus 6.8 months, HR=0.62, P<0.0001) and the PFS benefit was seen regardless of the PD-L1 status in all patients (5,11). The PFS benefit, however, was even more pronounced in patients expressing a T effector gene signature (11.3 versus 6.8 months, HR=0.51, P<0.0001) (5,6) indicative that the expression of gene signatures may be more robust to predict clinical response following treatment with PD-L1 inhibitors.
Although PD-L1 testing has clearly some limitations as a predictive biomarker, it is currently widely used (17). Despite progress made so far in terms of novel immunotherapy strategies for NSCLC, the major challenge still remains to identify those patients with advanced or metastatic NSCLC who will benefit the most following treatment with immune checkpoint inhibitors. In this regard the role of the putative novel predictive biomarkes evaluated in the CheckMate-227 and in the IMPower 150 trials may, if confirmed in further prospective clinical trials, offer a new perspective for predicting immunotherapy treatment outcomes of NSCLC patients in the near future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leal TA, Ramalingam SS. Immunotherapy in previously treated non-small cell lung cancer (NSCLC). J Thorac Dis 2018;10:S422-32. [Crossref] [PubMed]
- Dempke WC, Sellmann L, Fenchel K, et al. Immunotherapies for NSCLC: Are We Cutting the Gordian Helix? Anticancer Res 2015;35:5745-57. [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Reck M, Socinski MA, Cappuzzo F, et al. Primary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel +/- bevacizumab, with or without atezolizumab in 1L non-squamous metastatic NSCLC (IMPower150). Ann Oncol 2017;28:abstract LBA1_PR.
- Kowanetz M, Socienski MA, Zou W, et al. IMPower150: efficacy of atezolizumab (atezo) plus bevacizumab (bev) and chemotherapy (chemo) in 1L metastatic nonsquamous NSCLC (mNSCLC) across key subgroups. Proc Am Assoc Cancer Res 2018;78:abstract CT076.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Garassino MC, Vansteenkiste JF, Kim J, et al. Durvalumab in ≥ 3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. World Conference on Lung Cancer, 2016. abstract PL04a.03.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced non-squamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500-1. [Crossref] [PubMed]
- Jamieson NB, Maker AV. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther 2017;24:134-40. [Crossref] [PubMed]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]


