Current chemotherapy strategies in malignant pleural mesothelioma
Introduction
Chemotherapy is the mainstay of treatment of malignant pleural mesothelioma (MPM). The European Society for Medical Oncology (ESMO) guidelines, the National Comprehensive Cancer Network (NCCN) guidelines and the European Respiratory Society (ERS) guidelines indicate chemotherapy as an option for patients with ‘irresectable MPM’ who are not fit for major surgery (1-3). Only a minority of patients is fit enough to be a surgical candidate and the indication for surgery has become stricter in the last years.
Use of targeted therapy based on genetic profiling has been successful in other solid tumor types, targeting activating oncogenes. In MPM this approach has failed to improve clinical benefit in phase II studies. This is related to the fact that MPM is mostly driven by loss of tumor suppression genes like CDKN2A, NF2 and BAP1, rather than activation of oncogenes (4). Also, MPM is a heterogeneous tumor type (with three different subtypes) which makes it more challenging to develop effective therapies. Immunotherapy, targeting immune checkpoints (like PD-1 or its ligand PD-L1) has become standard of care in numerous solid tumors like non-small cell lung cancer (NSCLC) (5). The first studies with immunotherapy in second and third- line mesothelioma patients seem promising, but their value in the first line setting has yet not been defined (6-9). Therefore, chemotherapy remains a prominent treatment option in MPM.
In this review, the current available literature (see Table 1) and ongoing studies (see Table 2) with chemotherapy in the palliative setting and combination strategies in MPM are discussed. The use of chemotherapy as part of multimodality treatment and targeted therapy for MPM is covered in companion papers in this issue.
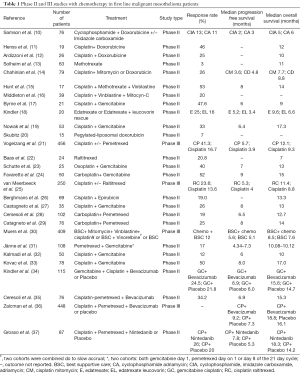
Full table
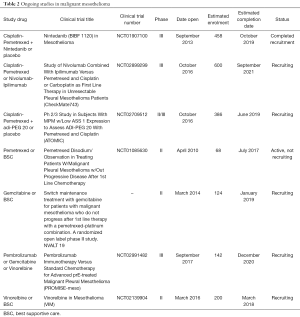
Full table
First line chemotherapy
For more than fifteen years, the standard first line treatment has been cisplatin- pemetrexed and it is currently the only regime approved by the Food and Drug Administration (FDA) for MPM. In the study by Vogelzang et al., 448 treatment naïve patients were 1:1 randomized to cisplatin monotherapy or cisplatin-pemetrexed doublet therapy, in which overall survival (OS) was the primary endpoint. Median OS in the cisplatin-pemetrexed arm was 12.1 vs. 9.3 months in the control arm (P=0.020, two-sided log-rank test). An updated analysis presented at the World Conference on Lung Cancer (WCLC) 2005 revealed a median OS for cisplatin alone of 9.0 and 12.8 months for the combination arm. Patients in the combination arm had a lower hazard ratio for death (0.77) compared with those in the control arm. The median time to progression was significantly longer in the cisplatin-pemetrexed arm; 5.7 vs. 3.9 months (P=0.001) and the response rates were higher (41.3%) in the cisplatin-pemetrexed arm versus 16.7% in the control arm (P<0.0001). The most common hematological toxicity in the cisplatin- pemetrexed combination arm was neutropenia (27.9% in the combination arm vs. 2.3% in the control arm). The most common non-hematological toxicities, in both groups, were nausea, vomiting and fatigue within around 90% of patients experiencing grade 3 toxicity. After 117 patients were enrolled, folic acid and vitamin B12 were added to reduce toxicity, resulting in a significant reduction in toxicities in the cisplatin-pemetrexed arm (21). More recently, the French Cooperative Thoracic Intergroup performed a phase III study, in which patients were 1:1 randomized to either cisplatin-pemetrexed or cisplatin-pemetrexed + bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor. The progression free survival (PFS) and OS were longer (7.3 and 16.1 months) in the cisplatin-pemetrexed arm compared to the study of Vogelzang. This improvement might be related to: use of rechallenge of pemetrexed, stricter inclusion criteria (like excluding patients with cardiovascular comorbidities) and the use of thoracoscopy as the diagnostic procedure which led to 90% efficient pleurodesis procedures (34). Replacement of cisplatin by carboplatin did not influence the PFS in patients with MPM, including similar 1-year survival rates (63.1% vs. 64.0%) and time to progression (7 vs. 6.9 months) (38).
The additional value of adding a (multitargeted) antifolate to cisplatin monotherapy was confirmed by a phase III study in 250 treatment naïve patients with MPM, comparing cisplatin vs cisplatin-raltitrexed. The combination therapy was superior to single agent therapy with a median survival of 11.4 months in de cisplatin-raltitrexed arm vs. 8.8 months for cisplatin alone, and the 1-year survival was 46% vs. 40% (P=0.048). There was a trend for a higher response rate in the combination arm (24% vs. 14%, P=0.06). Again, more patients experienced hematologic adverse events in the combination arm (neutropenia 16% vs. 8% in the combination and single agent arm respectively). Toxicity was the reason for holding back treatment in 23% of patients in the cisplatin-only arm and in 30% of patients in the combined arm. No toxic deaths were reported (25). The health-related quality of life was measured, and despite the toxicity of the treatment the quality of life was not affected and was equal in both treatment arms. Also, in both arms the dyspnea improved (39). Unfortunately, raltitrexed is not registered in many European countries for this indication.
Gemcitabine combined with a platinum compound, including cisplatin, carboplatin, and oxaliplatin has been tested in several phase II studies (see Table 1) (17,23,24,27,31,40). Response rates for these combinations have ranged from 12% to 50%, with acceptable levels of toxicity. However, it is generally accepted that mesothelioma patients should receive pemetrexed-based therapy in the first-line setting. Because gemcitabine is given on day 1 and 8 of a 3-week cycle and pemetrexed is given only on day 1 of a 3-week cycle, pemetrexed involves a lower frequency of hospital visits which benefits patients.
Despite the previous mentioned studies showing an improved (progression free) survival for combination strategies compared with single agent therapy, it was not compared with best supportive care (BSC). In the UK a study was conducted in the nineties to compare a BSC arm with two chemotherapy strategies, combining mitomycin, vinblastine, cisplatin and single-agent vinorelbine. Because of slow accrual, the two chemotherapy groups were combined. OS was compared as a primary outcome between both groups. This showed a trend towards better survival in the combination chemotherapy arms, even though chemotherapy schedules were used that are currently viewed as inferior (30).
Addition of angiogenesis inhibitors
Although platinum-pemetrexed are active agents in the first line treatment, only a minority of patients has clinical benefit. VEGF signaling is an important concept in mesothelioma cell pathophysiology (41). The addition of anti-angiogenesis agents to chemotherapy has been tested in several clinical studies. A phase II study by Ceresoli in 76 chemo-naïve MPM patients, receiving carboplatin-pemetrexed plus bevacizumab resulted in a response rate of 34.2%, a PFS 6.9 months and an OS of 15.3 months (35). The largest study was the phase III MAPS trial. The value of addition of maintenance bevacizumab, a VEGF antibody, was evaluated to first line cisplatin-pemetrexed therapy in 448 treatment naïve patients. PFS was significantly increased in the bevacizumab combination arm compared with patients who received cisplatin-pemetrexed alone [median 9.2 vs. 7.3 months; hazard ratio (HR) 0.61, 95% CI: 0.50–0.75]. Besides PFS, OS was also significantly increased with the combination (median 18.8 vs. 16.1 months; HR 0.77, 95% CI: 0.62–0.95). In the bevacizumab combination arm more grade 3 toxicity occurred, like hypertension (23% vs. 0%) and thrombotic events (6% vs. 1%) (36). A longer PFS and OS were seen in the MAPS trial compared to the phase II study of Ceresoli. It is unlikely that this is related to cisplatin-carboplatin switching. The selection of patients and the single arm set up might be responsible.
Addition of bevacizumab to cisplatin-gemcitabine in patients with MPM resulted in a similar response rate of 24.5% in the bevacizumab arm and 21.8% in the placebo arm. The median PFS and OS did not improve in the bevacizumab arm compared with the placebo arm (PFS 6.9 vs. 6.0 months, OS 15.6 vs. 14.7 months). There were no statistically significant differences in the rates of grade 3 or greater toxicity between treatment groups. Venous thrombosis developed in 17% of patients treated with the active agent and 9% on placebo (P=0.26) (34). It is still not clear why bevacizumab resulted in a survival benefit in combination with cisplatinum-pemetrexed and not with cisplatin-gemcitabine.
Nintedanib is a targeted therapy agent against i.e., the VEGF receptor. In the phase II LUME-Meso trial with chemo naïve patients with MPM, PFS was higher in the combination arm (cisplatin-pemetrexed-nintedanib median 9.4 months), compared to the cisplatin-pemetrexed-placebo arm median 5.7 months. Patients with a sarcomatoid type MPM were excluded in the trial. There was no survival benefit of nintedanib addition (30 vs. 32 months, P=0.319). There was an increased frequency of grade ≥3 toxicity linked neutropenia (43.2% vs. 12.2%), hypertension (9.1% vs. 2.4%) and diarrhea (6.8% vs. 0%) in the active agent arm. Three patients (6.8%) in the nintedanib arm and 7 patients (17.1%) in the placebo arm experienced AEs leading to permanent discontinuation of last study medication. No treatment related deaths were reported (37).
Both the LUME-Meso trial and the MAPS trial show an additional value of angiogenesis inhibition to platinum-pemetrexed. Despite the incoherent results of studies with the addition of angiogenesis inhibitors, the NCCN Panel now recommends adding bevacizumab to cisplatinum-pemetrexed as new first-line therapy option (1).
Addition of arginine depletion
Up to 63% of the MPM cells lack the argininosuccinate synthase 1 (ASS1), resulting in dependency on systemic arginine. Without arginine, cells will undergo apoptosis. In 82 MPM patients low ASS1 expression was found most frequently in the sarcomatoid type (7 out of 7) and less frequent in the mixed type (17 out of 25) and epithelial type (28 out of 50). A (possible) strategy to improve the survival in the first line treatment in MPM is the depletion of systemic arginine by using pegylated arginine deiminase (ADI-PEG) (42). To assess the clinical relevance of this mechanism in MPM, a phase 1 study of ADI-PEG with cisplatin-pemetrexed in patients in ASS1 deficient MPM patients (1 epithelioid, 2 biphasic, 2 sarcomatoid) was conducted. It showed a response in the two patients with the biphasic MM, in the epithelioid type and in 1 patient with a sarcomatoid type (43).
A randomized phase II study investigated single agent ADI-PEG. Patients with in ASS1-deficient MPM (both treatment naïve and pre-treated) were 2:1 randomized between ADI-PEG + BSC or BSC alone. Screening of 201 MPM patients identified 68 patients with low ASS1 expression (2 sarcomatoid, 66 non-sarcomatoid). The other patients were excluded because ASS1 was positive [83], ASS1 status could not be determined [21] or ASS1 was negative but other inclusion criteria were not met [29]. The median PFS was longer in the active agent arm compared to BSC alone (3.2 vs. 2.0 months, P=0.03). Half of the patients in the active agent arm experienced progression at the first 8-week tumor evaluation. The survival was equal between the two arms (11.1 BSC vs. 11.5 ADI-PEG). The toxicity profile was mild with 30% in the treatment arm experiencing a grade 3–4 event and 17% in the BSC arm (P=0.43) (44). So single agent ADI-PEG has a limited effect, and the efficiency of combination strategies with platinum-pemetrexed needs to be revealed.
Current studies
The PFS survival benefit of nintedanib to platinum-pemetrexed in the first line setting in the LUME-Meso trial warranted confirmation and the global, prospectively randomized phase III trial, which is currently awaiting its analyses (NCT01907100).
The first small phase II studies with immunotherapy in MPM patients in the second and third line seem promising (6-9). Based on these promising results with immunotherapy, the CheckMate743 (NCT02899299) is currently randomizing treatment naïve MPM patients to receive either platinum-pemetrexed or anti-PD1 (nivolumab) + anti-CTLA4 (ipilimumab). The estimated enrollment is 600 patients and the estimated primary completion is September 2021.
The ATOMIC phase II/III trial is currently recruiting sarcomatoid and biphasic MPM schould patients, in which patients are randomized between cisplatinum-pemetrexed plus ADI-PEG or placebo (NCT02709512) (45). The choice of excluding the epithelial type of MPM gives food for thought. A high proportion of ASS1 loss in the sarcomatoid type MPM was expected based on the retrospective series of Szlosarek (42). The phase II study of Szlosarek could only include two low ASS1 expression sarcomatoid MPM out of 201 screened MPM (44). Unfortunately, they did not separate the non-sarcomatoid group, so it is unknown how many mixed type MPM were ASS1 negative. So, it may be a challenge to recruit enough patients with a low ASS1 status, and also it might be hard to see if this is a right biomarker by excluding the epithelial type. Also, in the previous mentioned phase I study with only 5 MPM patients, there were partial responses in the sarcomatoid type (1 out of 2) and the biphasic type (2 out of 2), and epithelial type (1 out of 1). By excluding the epithelial type, one might miss clinical benefit for this group.
Several other combinations of targeted therapy with chemotherapy are under investigation and will be discussed in the companion paper in this issue including: The additional value of cetuximab to platinum-pemetrexed doublet therapy in first line setting (NCT00996567); the combination of gemcitabine and imatinib mesylate in pemetrexed-pretreated patients with malignant pleural mesothelioma (MPM) (NCT02303899); amatuximab in combination with pemetrexed and cisplatin (NCT02357147) and the combination of gemcitabine with ganetespib (NCT01590160).
Maintenance treatment
Current options
Unlike in other solid tumors like lung cancer, there is no current evidence for maintenance chemotherapy in MPM. Maintenance pemetrexed is feasible, but studies showing a better PFS or survival benefit are lacking. Single center experience with maintenance pemetrexed without progression on carboplatin- pemetrexed induction or pemetrexed monotherapy have been described. In a cohort of 13 patients (out of 30 patients who started with platinum-pemetrexed), patients were treated with pemetrexed maintenance therapy (PMT). The median survival in the maintenance group was 8.5 vs. 3.4 months in the cohort without maintenance therapy. Grade 3 toxicity consisted of neutropenia, leucopenia and anemia. The only non-hematological grade 3 toxicity during PMT was fatigue (15%). The reason to stop PMT was disease progression (69%), toxicity (23%) and in patient’s best interest (8%) (46).
The previous mentioned studies with cisplatin-pemetrexed with bevacizumab and nintedanib provide the first evidence for maintenance therapy with an anti-VEGF agent. In both studies maintenance anti-VEGF therapy was continued until disease progression after the initial 4–6 cycles of cisplatin-pemetrexed+ anti-VEGF (36,37). Other drugs after chemotherapy, like thalidomide (a well-known antiangiogenic agent), were tested in a large phase III study, randomizing patients to thalidomide or BSC. Unfortunately, no improvement was observed in progression free survival (3.6 months active agent arm vs. 3 in the BSC arm) (47).
Current studies
To determine the benefit of maintenance pemetrexed in MPM patients in patients without progression after first line platinum-pemetrexed doublet therapy, a randomized phase II study was designed (arm 1: pemetrexed, arm 2: BSC), with progression free survival as primary outcome. (NCT01085630). The study opened in April 2010, but no results have been presented yet.
Based on the advance of switch maintenance therapy in i.e., NSCLC and the previous activity of gemcitabine in phase II studies, a multi-center phase II study (NVALT 19) in The Netherlands is investigating switch maintenance therapy with gemcitabine in MPM patients without progression after platinum-pemetrexed doublet therapy and is currently open for randomization. Patients are 1:1 randomized to receive maintenance gemcitabine or BSC. The primary outcome is PFS and secondary outcomes are i.e., toxicity and OS. The first results are expected early 2019 (48).
Second line treatment
Current options
There is no standard second line treatment in MPM. The NCCN guidelines recommend consideration of rechallenge of pemetrexed (if not administrated in the first- line) if there was a good sustaining response at the time of initial chemotherapy interruption. Other options like vinorelbine, gemcitabine, and immunotherapy (pembrolizumab and nivolumab-ipilimumab) could be considered (1).
The additional value of pemetrexed in second line is doubtful. In a phase III study in 243 previous treated MPM patients (excluding pemetrexed) were patients randomized to BSC or pemetrexed. Pemetrexed prolonged the progression free survival (3.6 vs. 1.5 months, P=0.0148), although there was no effect on the primary endpoint OS (pemetrexed 8.4 vs. 9.7 in the BSC arm, P=0.7434). The authors suggested that this was due to the imbalance in post study therapies. Patients in the BSC were allowed to receive chemotherapy after discontinuation of the study. The percentage of BSC patients who received pemetrexed after discontinuation of study treatment was also much higher (18.3% vs. 3.3%, respectively; P=0.0001). Furthermore, BSC patients received chemotherapy, after discontinuation of the study, significantly earlier than P + BSC patients (median time to initiation, 4.3 vs. 15.7 months, respectively; log-rank P<0.0001) (49).
Manegold et al. analyzed whether the OS in the cisplatin-pemetrexed arm of the phase III study by Vogelzang et al. was influenced by post- study chemotherapy (PSC) (21,50). Less patients in the combination arm received PSC (37.2% vs. 47.3% in the cisplatin arm). The patients who received PSC had a survival benefit (P<0.01), but it is unknown whether this survival benefit is caused by the PSC, or that patients who lived longer received more second line treatment (50).
Predicting responses to chemotherapy would be of great value. A way to identifying the proper drug (combination) was developed by Schunselaar et al. With this technique, it is possible to perform a drug screening on primary mesothelioma cultures from pleural fluid and thereby guide treatment decisions of corresponding patients that were progressive after first or second line treatment. The in vitro prediction was adequate in seven out of the eleven drug screens. Limitation to this study was the inability to screen for pemetrexed sensitivity and the limited number of pleural fluid samples that led to a primary mesothelioma culture that was a candidate for drug screening (155 pleural fluid samples from 102 patients) (51).
Current studies
Currently, a randomized phase III study is investigating if immunotherapy (pembrolizumab) is beneficial compared to gemcitabine or vinorelbine in patients with progressive disease after at least one prior line of platinum-based chemotherapy. The estimated number of patients to be accrued is 142, with estimated completion of accrual end of 2020 (NCT02991482). Vinorelbine is currently also under investigation in a phase II study in MPM progressive patients after first line therapy. Patients will be randomised (1:2) to receive either BSC or BSC with vinorelbine (NCT02139904). The estimated enrolment is 200 patients and is expected to complete in March 2018.
The future of chemotherapy
Combining therapies like chemotherapy, targeted therapy and immunotherapy will be more and more prominent. The first studies in lung cancer, combining chemotherapy (like paclitaxel/carboplatin, platinum with gemcitabine or pemetrexed and docetaxel) with immunotherapy (like nivolumab with or without ipilimumab or pembrolizumab) are promising with improvement of PFS and response rates (52). Challenges will be to find the optimal combinations strategies in terms of timing, agents and to select the right patients for the right treatment.
Conclusions
Chemotherapeutic options have extensively been evaluated in the last three decades. This has resulted only in a few active chemotherapeutic regimes, which provide a limited but significant profit for the patients. A platinum-pemetrexed combination remains the standard first line therapy. There is growing evidence for addition of anti-angiogenesis therapy, like bevacizumab, to first line treatment. There is no standard second line treatment in which the value of single agent chemotherapy in recurrent seems limited. Combinations of active agents, including cytotoxic agents, targeted therapy and immunotherapy are currently under investigation, and first results seem promising. The next step is to reveal the optimal combination of chemotherapy with angiogenesis inhibitors or immunotherapy in the (near) future and to select the optimal treatment for the individual patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. JA Burgers: Advisory board AstraZeneca, Boehringer-Ingelheim, Roche Nederland. Prof. Dr. P Baas: Advisor/consultant BMS, MSD, AZ Pfizer and Grants of MSD and BMS. Dr. de Gooijer has no conflicts of interest to declare.
References
- National Comprehensive Cancer Network. Malignant Pleural Mesothelioma. NCCN Evidence Book. Version 2.2018.
- Baas p, Fennel D, Kerr KM, et al. Malignant Pleural Mesothelioma: ESMO Clinical Practice Guidelines. Ann Oncol 2015;26:v31-39.
- Scherpereel A, Baas P, Berghmans T, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264-9. [Crossref] [PubMed]
- Muller M, Schouten RD, De Gooijer CJ, et al. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther 2017;17:399-409. [Crossref] [PubMed]
- Disselhorst M, Harms E, Van Tinteren H, et al. OA 02.02 Ipilimumab and Nivolumab in the Treatment of Recurrent Malignant Pleural Mesothelioma: A Phase II Study. J Thorac Oncol 2012;11:S1746.
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Quispel-Janssen J, Zago G, Schouten R, et al. OA13.01 A Phase II Study of Nivolumab in Malignant Pleural Mesothelioma (NivoMes): with Translational Research (TR) Biopies. J Thorac Oncol 2017;12:S292-3. [Crossref]
- Scherpereel A, Mazieres J, Greillier L, et al. Second- or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Results of the IFCT-1501 MAPS2 randomized phase II trial. J Clin Oncol 2017;35:abstr TPS8507.
- Samson MK, Wasser LP, Borden EC, et al. Randomized comparison of cyclophosphamide, imidazole carboxamide, and adriamycin versus cyclophosphamide and adriamycin in patients with advanced stage malignant mesothelioma: a Sarcoma Intergroup Study. J Clin Oncol 1987;5:86-91. [Crossref] [PubMed]
- Henss H, Fiebig HH, Schildge J, et al. Phase-II study with the combination of cisplatin and doxorubicin in advanced malignant mesothelioma of the pleura. Onkologie 1988;11:118-20. [PubMed]
- Ardizzoni A, Rosso R, Salvati F, et al. Activity of doxorubicin and cisplatin combination chemotherapy in patients with diffuse malignant pleural mesothelioma. An Italian Lung Cancer Task Force (FONICAP) Phase II study. Cancer 1991;67:2984-7. [Crossref] [PubMed]
- Solheim OP, Saeter G, Finnanger AM, et al. High-dose methotrexate in the treatment of malignant mesothelioma of the pleura. A phase II study. Br J Cancer 1992;65:956-60. [Crossref] [PubMed]
- Chahinian AP, Antman K, Goutsou M, et al. Randomized phase II trial of cisplatin with mitomycin or doxorubicin for malignant mesothelioma by the Cancer and Leukemia Group B. J Clin Oncol 1993;11:1559-65. [Crossref] [PubMed]
- Hunt KJ, Longton G, Williams MA, et al. Treatment of malignant mesothelioma with methotrexate and vinblastine, with or without platinum chemotherapy. Chest 1996;109:1239-42. [Crossref] [PubMed]
- Middleton GW, Smith IE, O'Brien ME, et al. Good symptom relief with palliative MVP (mitomycin-C, vinblastine and cisplatin) chemotherapy in malignant mesothelioma. Ann Oncol 1998;9:269-73. [Crossref] [PubMed]
- Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol 1999;17:25-30. [Crossref] [PubMed]
- Kindler HL, Belani CP, Herndon JE, et al. Edatrexate (10-ethyl-deaza-aminopterin) (NSC #626715) with or without leucovorin rescue for malignant mesothelioma. Sequential phase II trials by the cancer and leukemia group B. Cancer 1999;86:1985-91. [Crossref] [PubMed]
- Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491-6. [Crossref] [PubMed]
- Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in mesothelioma. Cancer Invest 2002;20:693-9. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Baas P, Ardizzoni A, Grossi F, et al. The activity of raltitrexed (Tomudex) in malignant pleural mesothelioma: an EORTC phase II study (08992). Eur J Cancer 2003;39:353-7. [Crossref] [PubMed]
- Schutte W, Blankenburg T, Lauerwald K, et al. A multicenter phase II study of gemcitabine and oxaliplatin for malignant pleural mesothelioma. Clin Lung Cancer 2003;4:294-7. [Crossref] [PubMed]
- Favaretto AG, Aversa SM, Paccagnella A, et al. Gemcitabine combined with carboplatin in patients with malignant pleural mesothelioma: a multicentric phase II study. Cancer 2003;97:2791-7. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized Phase III Study of Cisplatin With or Without Raltitrexed in Patients With Malignant Pleural Mesothelioma: An Intergroup Study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Berghmans T, Lafitte JJ, Paesmans M, et al. A phase II study evaluating the cisplatin and epirubicin combination in patients with unresectable malignant pleural mesothelioma. Lung Cancer 2005;50:75-82. [Crossref] [PubMed]
- Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol 2005;28:223-6. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II Study of Pemetrexed Plus Carboplatin in Malignant Pleural Mesothelioma. J Clin Oncol 2006;24:1443-8. [Crossref] [PubMed]
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Jänne PA, Simon GR, Langer CJ, et al. Phase II trial of pemetrexed and gemcitabine in chemotherapy-naive malignant pleural mesothelioma. J Clin Oncol 2008;26:1465-71. [Crossref] [PubMed]
- Kalmadi SR, Rankin C, Kraut MJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: a phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 2008;60:259-63. [Crossref] [PubMed]
- Kovac V, Zwitter M, Rajer M, et al. A phase II trial of low-dose gemcitabine in a prolonged infusion and cisplatin for malignant pleural mesothelioma. Anticancer Drugs 2012;23:230-8. [Crossref] [PubMed]
- Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, Double-Blind, Placebo-Controlled, Randomized Phase II Trial of Gemcitabine/Cisplatin Plus Bevacizumab or Placebo in Patients With Malignant Mesothelioma. J Clin Oncol 2012;30:2509-15. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, Mencoboni M, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer 2013;109:552-8. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol 2017;35:3591-600. [Crossref] [PubMed]
- Santoro A, O'Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008;3:756-63. [Crossref] [PubMed]
- Bottomley A, Gaafa R, Manegold C, et al. Short-Term Treatment-Related Symptoms and Quality of Life: Results From an International Randomized Phase III Study of Cisplatin With or Without Raltitrexed in Patients With Malignant Pleural Mesothelioma: An EORTC Lung-Cancer Group and National Cancer Institute, Canada, Intergroup Study. J Clin Oncol 2006;24:1435-42. [Crossref] [PubMed]
- van Haarst JM, Baas P, Manegold Ch, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer 2002;86:342-5. [Crossref] [PubMed]
- Levin PA, Dowell JE. Spotlight on bevacizumab and its potential in the treatment of malignant pleural mesothelioma: the evidence to date. Onco Targets Ther 2017;10:2057-66. [Crossref] [PubMed]
- Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res 2006;12:7126-31. [Crossref] [PubMed]
- Beddowes E, Spicer J, Chan PY, et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1–Deficient Thoracic Cancers. J Clin Oncol 2017;35:1778-85. [Crossref] [PubMed]
- Szlosarek PW, Steele JP, Nolan L, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1–deficient malignant pleural mesothelioma: A randomized clinical trial. JAMA Oncol 2017;3:58-66. [Crossref] [PubMed]
- Szlosarek PW, Baas P, Ceresoli GL, et al. ATOMIC-Meso: A randomized phase 2/3 trial of ADI-PEG20 or placebo with pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient non-epithelioid mesothelioma. J Clin Oncol 2017;35:abstr TPS8582.
- van den Bogaert DP, Pouw EM, van Wijhe G, et al. Pemetrexed Maintenance Therapy in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol 2006;1:25-30. [Crossref] [PubMed]
- Buikhuisen WA, Burgers JA, Vincent AD, et al. Thalidomide versus active supportive care for maintenance in patients with malignant mesothelioma after first-line chemotherapy (NVALT 5): an open-label, multicentre, randomised phase 3 study. Lancet Oncol 2013;14:543-51. [Crossref] [PubMed]
- Janssen JQ, Baas P, Buikhuisen W, et al. pp02.36: switch-maintenance with gemcitabine for patients with malignant mesothelioma: feasibility of a randomized phase II study. Abstract book 13th international conference of the international mesothelioma interest group, 2016. Available online: http://imig2016.org/wp-content/uploads/2016/04/iMig-2016-Abstract-Book.pdf
- Jassem J, Ramlau R, Santoro A, et al. Phase III Trial of Pemetrexed Plus Best Supportive Care Compared With Best Supportive Care in Previously Treated Patients With Advanced Malignant Pleural Mesothelioma. J Clin Oncol 2008;26:1698-704. [Crossref] [PubMed]
- Manegold C, Symanowski J, Gatzemeier U, et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol 2005;16:923-7. [Crossref] [PubMed]
- Schunselaar LM, Quispel-Janssen JMMF, Kim Y, et al. Chemical profiling of primary mesothelioma cultures defines subtypes with different expression profiles and clinical responses. Clin Cancer Res 2018;24:1761-70. [Crossref] [PubMed]
- Dammeijer F, Lau SP, van Eijck CHJ, et al. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev 2017;36:5-15. [Crossref] [PubMed]


