Lung cancer screening with low-dose CT: a world-wide view
Introduction
Lung cancer is the leading cause of cancer death worldwide, comprising almost 20% of all such deaths (1). The concept of using low-dose computed tomography (LDCT) to screen for lung cancer dates back almost three decades (2). Early observational studies on high-risk but asymptomatic subjects showed that LDCT detected more lung cancers than chest radiography, and that many of the detected cancers were early stage (3,4). The promising observational data led to the launching of several generally small randomized trials of LDCT screening, mainly in Europe (5-11). Additionally, they led to a definitive study in the USA, the National Lung Screening Trial (NLST), a randomized trial of over 50,000 high-risk current and former smokers comparing LDCT screening to screening with chest radiography (12). In 2011, the NLST reported a statistically significant 20% lung cancer mortality reduction in the LDCT arm (13).
The finding of a mortality reduction in NLST, however, was tempered by the high false positive rate seen in the trial, around 25% in the first two rounds (13). Concerns about the potential harms of false positive screens and downstream invasive diagnostic procedures in a proposed screening eligible population with high rates of comorbidities, as well as some concerns about radiation dose and screening program costs, resulted in a slow uptake of LDCT screening in the USA and elsewhere (14,15). To date, there are no nationwide, population-based programs of LDCT lung cancer screening, as there are for cervical, colorectal and breast cancer screening. There have been, though, in addition to a number of randomized trials and observational studies, various demonstration projects of LDCT screening worldwide.
In this article, we first present comparative global rates of lung cancer mortality, as well as rates of cigarette smoking. Next, we perform a world-wide regional review of randomized controlled trials (RCTs) and demonstration projects of LDCT screening. Finally, we review LDCT screening guidelines and position papers from various countries and regions.
Global lung cancer mortality rates and cigarette smoking rates
Worldwide lung cancer mortality rates are available from GLOBOCAN 2012, a World Health Organization (WHO) resource (1). There were an estimated 1.6 million deaths from lung cancer in 2012, with about 70% of these occurring in men. By WHO regions, about 40% of lung cancer deaths (627,000) were in more developed regions (USA, Canada, Europe, Australia/New Zealand and Japan), 37% (597,000) were in China, and the remaining 23% were in other less developed regions. Table 1 shows mortality rates for men and women by WHO region. Among men, lung cancer mortality rates (age-standardized, per 100,000) were highest in Eastern Asia and Central and Eastern Europe, with a rate of about 45, followed by Southern Europe with a rate of 40 and Northern America (USA & Canada), Western Asia and Western Europe, with rates of around 35. Rates in most of Africa, South-Central Asia, South America and Central America were 20 or lower. For women, rates were highest in Northern America, 25, and Northern Europe, 20, followed by Eastern Asia and Western Europe, with rates of about 15. Rates were 10 or less in Africa, South-Eastern Asia, South and Central America, Western Asia, Southern Europe, and Central and Eastern Europe.
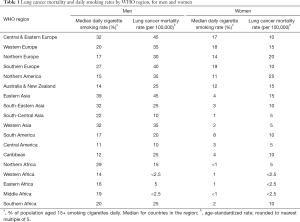
Full table
Comparative daily cigarette smoking rates (proportion of the population 15 and over reporting that they smoke daily) are also available from WHO (Table 1) (16). Smoking rates generally correlate with lung cancer mortality rates. Daily smoking rates for men were highest in Asia, with rates in East, South-Eastern and Western Asia in the range of 32–39%. Northern America and Australia/New Zealand had relatively low rates, along with Central America (range, 11–15%). For women, rates were lower than for men in all regions, with rates under 10% in 12 of the 18 listed regions; the highest rates were in the European regions (range, 14–19%).
Randomized trials and demonstration projects around the world
North and South America
USA
The National Lung Screening Study (NLST) was a multi-center randomized trial of annual screening (over three rounds) with LDCT versus chest-radiograph (CXR) in high-risk subjects (12). Inclusion criteria were age 55–74, at least 30 pack-years of smoking, and for former smokers, having quit within 15 years. A positive LDCT screen was defined as a non-calcified nodule of at least 4 mm in diameter.
A total of almost 55,000 men and women were enrolled in NLST (13). LDCT screen positivity rates were around 27% in the first two rounds, and 16% in the third round (in the third round, a nodule stable over two years could be counted as a negative screen on the discretion of the radiologist). The reported risk ratio for lung cancer mortality was 0.80 (95% CI: 0.73–0.93); there was also a significant reduction in all-cause mortality. In the LDCT arm, 649 of 1,060 lung cancers were screen detected; 63% of LDCT screen detected cancers and 50% of all cancers were stage I (A or B).
The NLST was proceeded by a pilot trial, the Lung Screening Study (LSS), which was designed to assess the feasibility of performing a large screening trial such as the NLST (17). The pilot study demonstrated high compliance with LDCT and CXR screening and low cross-over rates (subjects randomized to CXR receiving LDCT screening or vice-versa).
Canada
In 2000, the British Columbia Cancer agency launched a LDCT screening demonstration project (18). Volunteer current and former smokers aged 50–74 with 30+ pack-years were recruited for the study. A total of 561 subjects were enrolled and completed a baseline LDCT screen. Those with an abnormal baseline screen (at least one non-calcified nodule) were followed with serial CT scans until resolution of nodule status; subjects with a normal baseline screen were not followed. The abnormal screen rate was 46% (259/561). Twenty-one lung cancers were detected by screening, for a yield of 3.7% (21/561); 16 of these were seen at baseline and 5 first seen on follow-up scans. Of the 21, 14 (67%) were stage I.
In Ontario, Cancer Care Ontario (CCO) launched a pilot organized lung cancer screening program in 2017 (19). Four screening center sites (hospitals) in Ontario were selected for participation. The pilot will be evaluated to assess key components of the screening pathway, including recruitment, navigation, retention, follow-up, cancer stage at diagnosis and treatment. Results of the pilot evaluation will inform the design and implementation of a provincial lung cancer screening program. The pilot will provide navigation support to participants throughout their screening journey, as well as smoking cessation support. Persons assessed as having a two percent or greater risk of developing lung cancer over the next six years will be considered eligible to participate.
Brazil
The First Brazilian Lung Cancer Screening Trial (BRELT1) was initiated in 2013 in greater Sao Paulo (20). The inclusion criteria were essentially those of the NLST, specifically, age 55–74, 30+ pack-years of smoking and having quit within the past 15 years for former smokers. All subjects were offered a one-time LDCT screen. Scans with indeterminate pulmonary nodules greater than 4 mm were considered positive screens.
A total of 4,030 individuals applied for the screening study, of which 864 met the inclusion criteria and 790 eventually agreed to participate. At baseline, 312 (39.5%) of subjects had a positive screen; a total of 552 nodules larger than 4 mm were recorded. For a cutoff size of 6 mm or larger, 145 (18.4%) subjects had a positive screen. Among the 312 positive screens, lung cancer was detected in 10 subjects (3.2%); the lung cancer yield was 1.3% (10 of 790). Eight of the ten cases were stage 1A or B.
Europe
Italy
In Italy, a small LDCT screening demonstration project began with the new century and was followed by three small mortality endpoint RCTs.
A demonstration screening program in Lombardy began in 2000 with a pilot study enrolling 1,035 volunteers electing to receive annual LDCT on a long-term basis (5). Eligibility criteria were current or former smokers aged 50 or over with at least 20 pack-years of smoking. The low-dose protocol was 140 kV, 40 mA and 10 mm collimation using a GE CT Hispeed scanner; effective radiation dose was 0.7 mSv. Non-calcified nodules >5 mm in maximum diameter were considered suspicious and requiring of follow-up. At baseline 4.4% of subjects had a nodule >5 mm; the lung cancer yield among all those screened was 1.1%.
The same group organizing the above program initiated the MILD (Multicentric Italian Lung Detection) randomized trial in 2005; it compared annual or biennial LDCT with a control arm (5). Eligibility criteria were age 49 or above, at least 20 pack-years of smoking and current smoking or having quit within 10 years. A total of 4,099 participants were randomized. The trial was originally planned for a sample size of 10,000 at multiple centers; however, various obstacles, including lack of funding and a lack of local support limited the trial’s scope to one screening center, in Milan. Subjects in the annual (N=1,190) and biennial (N=1,186) arms received a median of 5 and 3 screens, respectively. The recall rates at baseline were 14% and 15% in the biennial and annual arms, respectively. Of 49 total screen detected cancers in the annual and biennial arms (combined), 32 (65%) were stage I. At 5 years follow-up, there were seven, six, and twelve lung cancer deaths in the control, biennial and annual arms, respectively, corresponding to rates (per 100,000 PY) of 108.5, 108.8 and 216.0, respectively.
The DANTE trial began recruitment in Milan (Lombardy) in 2001 with the goal of comparing LDCT screening over 5 annual rounds to a control arm (6). Eligibility criteria were aged 60–74 and at least 20 pack-years of smoking. All subject received a baseline CXR and 3-day sputum cytology testing. Screened arm subjects also had a baseline LDCT scan the same day. Both arms underwent an annual physical exam and medical interview. Approximately 2,500 subjects were enrolled, 1,264 LDCT and 1,186 control. Long-term results showed a lung cancer mortality HR of 0.99 (95% CI: 0.69–1.4), with lung cancer mortality rates in each arm of about 540 per 100,000 PY; median F/U was 8.4 years. Of lung cancers in the screened arm, 29/104 were detected on the baseline LDCT screen and 37/104 on post-baseline LDCT screens. Of all lung cancers, 45% were stage I in the LDCT arm, compared to 22% in the control arm. The proportion of all subjects with any lung cancer and stage III/IV lung cancer was 8.2% and 3.4%, respectively, in the LDCT arm and 6.1% and 3.8% in the control arm.
The Italung Trial was launched in 2004 at three centers in Tuscany (7). A total of 3,206 subjects (1,613 screened arm, 1,593 control arm) were randomized to either annual LDCT over 4 rounds or to no screening (control). Eligibility criteria were age 55–69, at least 20 pack-years and current smoking or having quit within 10 years. Lung cancer was diagnosed in 67 screening arm and 71 control arm subjects, with 36% of screening arm and 11% of control arm cancers being stage I. There were 43 lung cancer deaths in the screening arm versus 60 in the control arm (RR =0.70, 95% CI: 0.47–1.03).
France
A pilot trial of LDCT screening was launched in 2002 in France (8). Subjects were recruited from the practices of general practitioners and occupational physicians. Subjects were randomized to an LDCT or a CXR arm. Eligibility criteria were age 50–75, current smokers or former smokers having quit within 15 years, and cigarette consumption of at least 15 cigarettes per day for at least 20 years.
A total of 385 and 380 subjects were randomized to LDCT and CXR arms, respectively. Compliance with the baseline screen was 86% for LDCT and 75% for CXR. At least one non-calcified nodule (NCN) was observed on the baseline LDCT screen in 152 (45.2%) subjects; nodules ≥5 to 10 mm and ≥10 mm were observed in 53 (15.8%) and 28 (8.3%) subjects, respectively. Lung cancer was detected in 8 (2.4%) subjects at the baseline LDCT screen and in one subject (0.4%) at the baseline CXR screen.
Denmark
In Denmark, a randomized trial of LDCT screening, the Danish Lung Cancer Screening Trial (DLCST) was initiated in 2004 (9). The trial enrolled approximately 4,000 subjects aged 50–70 with at least 20 pack-years, with former smokers needing to have quit within the past 10 years. Screening arm subjects received 5 annual LDCT screens; control arm subjects received no screening. Participants with nodules between 5 and 15 mm without benign characteristics were re-scanned at 3 months; those with nodules larger than 15 mm or rapidly growing nodules (>25% increase in volume) were referred to pulmonologists for work-up.
The mean annual participation rate in the LDCT arm was 95.5%. The baseline recall rate for a follow-up scan was 7.6%, with annual rates in the following years ranging from 0.9% to 1.3%. A total of 198 participants were referred for diagnostic evaluation by pulmonologists over all rounds (average 2.0 per round). Significantly more total lung cancers and early stage lung cancers (stage I/II) were found in the screening arm (100 and 54, respectively) as compared to the control arm (53 and 10, respectively). There was an essentially equal number (and rate) of lung cancer deaths in each arm (39 screening arm vs. 38 control arm).
Netherlands/Belgium
The Dutch-Belgian Lung Cancer Screening Trial (NELSON) is a randomized trial, launched in 2003, comparing LDCT screening with usual care (10). The primary outcome is lung cancer mortality through 10 years. The eligibility criteria were age 50–75 years, and having smoked either ≥15 cigarettes per day for at least 25 years or ≥10 cigarettes per day for at least 30 years. In addition, former smokers had to have quit within the past 10 years. The original protocol specified LDCT screens at baseline and year 1 and 3; a fourth screening round at year 5.5 was subsequently included. Screen outcomes were based on volumetric measurements. For newly detected solid nodules, the volume categories of <50, 50–500 and >500 mm3 were classified as negative, indeterminate and positive, respectively. For previously detected nodules, the outcome classification was based on nodule volume doubling time.
A total of 15,822 individuals were randomized, 7,915 to the LDCT arm and 7,909 to the no screening arm (10). Compliance rates with LDCT screening across the first three rounds ranged from 95.5% (baseline) to 87.5% (round 3). At baseline, 19.2% of subjects had an indeterminate screen result, and 1.6% a positive result. Across the four screening rounds, there were 255 screen-detected lung cancers, with 69% being stage I (A or B) (21).
The lung cancer mortality results for NELSON are expected to be reported sometime in 2018.
Germany
The German Lung Cancer Screening Intervention Trial (LUSI) began in 2007 (22). LUSI randomized individuals aged 50–69 with a history of heavy smoking. Screened arm subjects received five annual rounds of LDCT screening; control arm subjects received no screening. A total of 4,052 participants were randomized, from the Heidelberg area. A newly observed (first or subsequent screening round) nodule ≥5 mm was considered a positive screen. A previously noted nodule constituted a positive screen if it was >10 mm or had a volume doubling time (VDT) of <600 days. At the baseline screen, 22.2% had a positive screen. At later screening rounds, the positivity rate ranged from 4.0–5.7%. The cancer detection rate was 1.1% at baseline and ranged from 0.4% to 0.6% at the subsequent screening rounds. Of screen detected cancers, 72% (42/58) were stage I.
United Kingdom
The UK Lung Cancer Screening Trial (UKLS) was a randomized pilot trial designed to assess the effectiveness in risk prediction modelling to select individuals for LDCT screening, to evaluate the use of volumetric analysis in the management of CT-detected nodules, and to determine the cost-effectiveness of LDCT screening (23). Starting around 2011, questionnaires were sent to almost 250,000 individuals aged 50–75 identified from population-based Primary Care Trust records for given areas of the UK. Of 247,354 persons sent questionnaires, 75,958 (30.7%) responded as willing to participate, and of these, 8,729 (1.5%) were classified as high risk (≥5% risk of lung cancer in 5 years) by the Liverpool Lung Project (LLP) risk model. Of the 8,729, 4,055 individuals eventually consented and were included in the trial; 2,028 in the screened arm and 2,027 in the control arm.
The participation rate for (baseline) CT screening was 98%. The lung cancer detection rate from the screen was 2.1% (1.7% at baseline and 0.4% within 12 months with follow-up CT). Of 42 screen detected cancers, 28 (66.7%) were stage I and 8 (19%) were stage II. The false positive rate, defined as category 3 (volume 50–500 mm3 or 5–9.9 mm diameter for solid nodules) or category 4 (volume >500 mm3 or diameter ≥10 mm) findings requiring further evaluation before 12 months (in those without lung cancer), was 27%; this was divided into 3.6% referred either initially or after repeat scanning to a multidimensional team and 23.2% only referred for further interval CT imaging. A full planned follow-up trial of 28,000 subjects was not funded.
Asia
Japan
Some of the earliest studies of low-dose CT lung cancer screening world-wide were performed in Japan. In 1993, the Anti-Lung Cancer Association (ALCA) introduced LDCT screening to its members, most of whom were men aged 50 or older with at least 20 pack-years of smoking (3). Previously, ALCA members had received lung cancer screening with chest radiography and sputum cytology. From 1993 to 1995, 1,369 members received one or more LDCT scans (average 2.5). Abnormalities were present on 10.1% of LDCT scans. There were 15 LDCT detected lung cancers, with 14 being stage I.
In Hitachi City Japan, a LDCT screening program was launched by Hitachi Health Care Center in 1998 for employees, retirees and spouses aged 50–69 (23). In 2001, Hitachi Medical Center initiated LDCT screening for city residents 50 years and older (24). In both programs, smoking history was not a consideration for eligibility. As reported through 2006, there were 61,914 CT screenings performed in 25,385 individuals. The lung cancer detection rate in the baseline screen was 0.97% (Hitachi Medical Center) and 0.42% (Hitachi Health Care Center); on repeat screening detection rates were 0.46% (Medical Center) and 0.07% (Health Care Center). Only 40% of those with screen detected lung cancer were current or former smokers (76% of men and 6% of women); 91% of screen detected cancers were stage I. By 2009, an estimated 40% of city residents aged 50–69 had undergone CT screening at least once.
South Korea
A lung cancer screening program with LDCT was launched in Korea in 1999 among asymptomatic individuals aged 45 and older at Samsung Medical Center (25). Over a 4-year period, 6,406 persons were screened with LDCT one to four times, with a total of 10,932 (mean 1.7) screens. Of the 6,406, 52% were high-risk (≥20 pack-years) ever-smokers, 25% were non-high risk ever smokers and 23% were never smokers. On LDCT, 35% of subjects had at least one non-calcified nodule. A total of 23 lung cancers were detected on LDCT screening (11 at baseline and 12 during repeat screening), giving a detection rate of 0.36% (23/6,406) on an individual basis. Of the 21 non-small cell lung cancers detected, 62% (N=13) were stage 1, with the rest stage IIA (N=1), stage IIIA/B (N=5) and stage IV (N=2); 14 presented as solid nodules and 7 as ground-glass opacity (GGO) nodules.
China
In 2010, as part of national cancer screening demonstration program, LDCT screening was initiated in three regions with high lung cancer mortality rates, Xuanwei City (Yunnan Province), Dagang Oilfield (Tianjin Province) and Haidan District (Beijing) (26). Over a 3-year period, 8,145 individuals were screened. The detection rate at the baseline screen was 1.68%; however, only 34% of the 137 cases were stage I.
In 2012, the Chinese government launched a cancer screening project for the five top cancers in selected urban areas of the country, with LDCT used for lung cancer screening (26). An estimated 210,000 subjects will receive free baseline LDCT screenings from 2012-2017 under this program. In addition, in 2013, the Beijing municipal government launched a LDCT screening program aimed at establishing and optimizing a LDCT screening protocol; it is expected to enroll over 8,000 participants over three years.
In 2014, the Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS) launched a pilot trial of low-dose CT screening (27). Approximately 2,700 high-risk subjects at three provincial centers (Changsha City, Hunan Province; Lanzhou City, Gansu Province; Haining City, Zhejiang Province) were randomized into three arms: annual LDCT screening for three years, biennial LDCT screening (screening at years 1 and 3), and no LDCT screening. Trial subjects also received screening for colorectal cancer. The adherence rate for LDCT screening at baseline was 89%. The rates of baseline LDCT screens suspicious for lung cancer were 6.5% and 6.1% in the annual and biennial LDCT arms, respectively.
The pilot study was undertaken to assess the feasibility of performing a full-scale trial of LDCT screening in China. Planning for such a trial is currently ongoing.
Taiwan
In 2012 a demonstration LDCT screening program was undertaken for one year at one hospital in the setting of yearly medical examinations (28). Asymptomatic adults with no history of any cancer were eligible for screening; a history of smoking was not required. A baseline CT scan with any non-calcified nodule ≥4 mm in diameter was defined as a positive screen.
A total of 3,339 individuals had a baseline LDCT screen in the program. The mean age was 48 (range, 19–86), with 25% current smokers, 27% former smokers and 49% never smokers. The positivity rate for baseline LDCT was 38.3%. A total of 34 lung cancers were detected on LDCT screening (cancer detection rate of 1.0%). Of the 34 cancers, 30 (88%) were adenocarcinomas, 3 were invasive thymoma, and 1 was a germinoma. Of the 30 adenocarcinomas, 12 (40%) were in-situ, 16 (53%) were stage IA, 1 was stage IB, and 1 was stage III.
Guidelines and position statements
North America
USA
The United States Preventive Services Task Force (USPSTF), an independent entity authorized by the United States Congress to make recommendations on preventive health services, issued its recommendation for lung cancer screening in 2013 (29). It recommended annual LDCT screening for persons aged 55–80 meeting the NLST smoking history criteria (i.e., ≥30 pack-years and either a current smoking status or having quit within 15 years). The National Comprehensive Cancer Network (NCCN) recommends engaging in shared-decision making about LDCT lung cancer screening, including discussion of benefits and risks, for subjects aged 55–74 meeting the NLST smoking history criteria (category 1) and for subjects aged 50 and over with ≥20 pack-years of smoking and additional risk factors that increase the 6-year lung cancer risk to ≥1.3% (category 2) (30). The American Cancer Society, a patient advocacy and research organization, recommends annual LDCT screening for persons aged 55 to 74 meeting the NLST smoking history criteria (31).
Canada
The Canadian Task Force on Preventive Health Care issued its guidelines on LDCT lung cancer screening in 2016 (32). It recommended LDCT screening for subjects aged 55–74 years meeting the NLST smoking history criteria. The recommendation was for annual screening up to three consecutive times and was stated as a weak recommendation, based on low quality evidence. In addition, the recommendation stated that screening should only be carried out in health care settings with expertise in early diagnosis and treatment of lung cancer. For other adults not meeting the above criteria based on age or smoking history, the task force gave a recommendation for not screening for lung cancer with LDCT.
European Union (EU)
In late 2017, the EU issued a position statement describing the current status of lung cancer screening and identifying essential elements for a successful European lung cancer screening program (33). The statement was composed by an expert group from eight European countries comprising all of the specialties and professions involved in lung cancer screening and follow-up.
The position statement contained ten recommendations related to implementing lung cancer screening in Europe. The recommendations include the following: (I) that low-dose CT is the only evidence-based method for the early detection of lung cancer shown to provide a mortality reduction; (II) that a validated risk stratification approach be used to select who should be screened; (III) that the potential benefits and harms should be discussed and smoking cessation advice offered; (IV) that management of nodules should use volumetric measurements; (V) that national quality assurance boards be set up by professional bodies; (VI) that management of nodules detected in different settings (prevalent screen, incident screen, clinical practice) should be managed with different protocols due to different pre-test lung cancer risk; (VII) that a personalized approach to lung cancer screening be considered; (VIII) that management of lung nodules should be done by multidisciplinary teams with the aim of minimizing harm and ensuring patients receive the most appropriate treatment; and (IX) that planning for low-dose CT screening should be started throughout Europe because it has the potential to save lives.
Asia
Japan
In 2013, the Japanese Imaging Guideline was published by the Japan Radiological Society and the Japanese College of Radiology (34). With respect to lung cancer screening, for persons aged 50 or over with a Brinkman index ≥600 (i.e., ≥30 pack-years of smoking), the guidelines state that while the evidence in insufficient, LDCT screening may be considered as a measure for population-based screening. For the non-high-risk group, LDCT screening is not recommended.
Conclusions
Most major countries in the developed world have initiated research on LDCT lung cancer screening, generally in the form of small RCTS or regional demonstration projects. These efforts have sought to establish the feasibility of performing LDCT screening in the respective countries, and also to add to the overall knowledge base of lung cancer screening with LDCT. In North America and Europe, the studies generally involved only high-risk smokers, while in East Asia the studies often included some low-risk smokers or never smokers, along with high-risk smokers.
To date, no country has established a nationwide organized LDCT lung cancer screening program. In Europe, many countries are awaiting the results of the NELSON trial, the other large-scale LDCT screening trial, in addition to the NLST (15). In China, the recent pilot trial was designed to help design a definitive trial in the country, the results of which, if undertaken, may determine whether large-scale LDCT screening programs are established there. In the USA, LDCT screening is generally covered by private or government (Medicare) insurance for the screen-eligible population; however, the dissemination of LDCT screening has been slow, with currently under 5% of those eligible being screened (35).
Outside of the USA, cancer screening is typically performed through organized programs at the national or provincial level, utilizing population-based invitations to screening. A special challenge of implementing an organized, population-based program of screening for lung cancer, as opposed to breast, cervical or colorectal cancer, is that the eligibility criteria go beyond age and sex, and include characteristics not readily identifiable from national population registries, specifically, details about smoking history and current smoking status (15,36). Other challenges include how to integrate smoking cessation programs into LDCT screening, how to manage the additional radiological services required for screening without compromising existing critical needs, and how to deal with ancillary findings on LDCT scans (15,36).
Successful tobacco control remains the most important priority internationally for reducing the world-wide burden of lung cancer in the medium- to long-term timeframe. Countries with moderate or high levels of cigarette smoking should devote adequate resources to this task and promote policies that will reduce national smoking rates. However, such efforts will have little effect in the short-term (next 10–15 years) on that proportion of the population who still smoke or who have recently quit and who remain at high risk for lung cancer mortality. To modestly mitigate the risk of dying from lung cancer in this segment of a country’s population, implementing LDCT screening, with necessary quality controls and monitoring, and in the appropriate high-risk populations, may be a reasonable option for those nations whose overall resources and health care infrastructure are adequate for the task.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Naidich DP, Marshall CH, Gribbin C, et al. Low-dose CT of the lungs: preliminary observations. Radiology 1990;175:729-31. [Crossref] [PubMed]
- Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. [Crossref] [PubMed]
- Henschke CI, McCauley D, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. Long-term follow-up results of the DANTE Trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015;191:1166-75. [Crossref] [PubMed]
- Paci E, Puliti D, Pegna AL, et al. Mortality, survival and incidence rates in the ITALUNG randomized lung cancer screening trial. Thorax 2017;72:825-31. [Crossref] [PubMed]
- Blanchon T, Brechot J, Grenier P, et al. Baseline results of the Depiscan study: A French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Vliegenthart R, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659-67. [Crossref] [PubMed]
- Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9-15. [Crossref] [PubMed]
- The National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [Crossref] [PubMed]
- The National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Raz DJ, Wu GX, Consunji M, et al. Perceptions and utilization of lung cancer screening among primary care physicians. J Thorac Oncol 2016;11:1856-62. [Crossref] [PubMed]
- van der Aalst CM, ten Haaf K, deKoning HJ. Lung cancer screening: latest developments and unanswered questions. Lancet Respir Med 2016;4:749-61. [Crossref] [PubMed]
- World Health Organization. WHO report on the global tobacco epidemic 2015. Available online: http://www.who.int/tobacco/global_report/2015/en/
- Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT vs chest radiograph: The Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21. [Crossref] [PubMed]
- McWilliams AM, Mayo JR, Ahn MI, et al. Lung cancer screening using multi-slice thin-section computed tomography and autofluorescence bronchoscopy. J Thorac Oncol 2006;1:61-8. [Crossref] [PubMed]
- Cancer Care Ontario. Guidelines & Advice: Lung cancer screening pilot for people at high risk. Available online: https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/lung-cancer-screening-pilot-people-at-high-risk
- dos Santos RS, Franceschini JP, Chate RC, et al. Do current lung cancer screening guidelines apply for populations with high prevalence of granulomatous disease? Results from the First Brazilian Lung Cancer Screening Trail (BRELT1). Ann Thorac Surg 2016;101:481-6; discussion 487-8. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany. J Thorac Oncol 2015;10:890-6. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Nawa T, Nakagawa T, Mizoue T, et al. Low-dose computed tomography screening in Japan. J Thorac Imaging 2015;30:108-14. [Crossref] [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 2005;20:402-8. [Crossref] [PubMed]
- Zhao SJ, Wu N. Early detection of lung cancer: low-dose computed tomography screening in China. Thoracic Cancer 2015;6:385-9. [Crossref] [PubMed]
- Hu P, Dai M, Shi J, et al. The feasibility study of a randomized cancer screening trial in China [abstract]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res 2016;76: Abstract nr 1795.
- Chen CY, Chen CH, Shen TC, et al. Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J Formosan Med Assoc 2016;115:163-70. [Crossref] [PubMed]
- Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med 2014;160:330-8. [PubMed]
- National Comprehensive Cancer Network. Lung Cancer Screening. Version 2.2018 – August 8th, 2017. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf
- American Cancer Society. American Cancer Society guidelines for the early detection of cancer. Available online: https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
- Canadian Task Force on Preventive Health Care. Lung Cancer (2016). Available online: https://canadiantaskforce.ca/guidelines/published-guidelines/lung-cancer/
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- Japan Radiologic Society. The Japanese Imaging Guideline, 2013. Available online: http://www.radiology.jp/english/guideline.html
- Pinsky PF. Does the evidence support the implementation of lung cancer screening with low-dose CT? Expert Rev Respir Med 2018;12:257-60. [Crossref] [PubMed]
- Field JK, Duffy SW, Devaraj A, et al. Implementation planning for lung cancer screening: five major challenges. Lancet Respir Med 2016;4:685-7. [Crossref] [PubMed]


