Extended pleurectomy decortication: the current role
Definitions
The confusion and disagreement regarding the different procedures performed for malignant pleural mesothelioma (MPM) has been clarified (1), and they now should be named according to the agreed definitions. Previously pleurectomy/decortication (P/D) comprised a heterogeneous group of procedures ranging from extensive biopsies to ‘radical’ intent procedures. Now it has been established that pleurectomy decortication has the intent of removing all the macroscopic disease, with the aim of prolonging survival. P/D is total parietal and visceral pleurectomy, sparing the pericardium and the hemidiaphragm, while extended pleurectomy/decortication (EPD) is the above plus the resection of the pericardium and the hemidiaphragm, when required, and in order to remove all the macroscopic disease. Extra-pleural pneumonectomy (EPP) is the en bloc removal of the visceral and parietal pleura, lung, hemidiaphragm and pericardium of the affected hemithorax.
What are the technical areas of debate in P/D?
In the few cases of early disease that one encounters in mesothelioma surgery, there remains debate around how extensive the resection should be. One argument proposes the most radical resection in the form of EPP. This is supported by the survival data from the IASLC staging project which showed longer survival from stage I disease after EPP rather than P/D (2,3). There is the persuasive argument that the lack of accurate nodal sampling in P/D (no intrapulmonary nodes are removed) caused a falsely low survival for apparent stage I due to stage migration. The contrary argument is that total visceral pleurectomy is not indicated in the apparent absence of macroscopic disease. The surgeon must balance the risk of minor residual disease against the risk from prolonged air leak.
The control of parenchymal air leak remains one of the greatest challenges of P/D. Many technical innovations have been proposed to remove the visceral layer without excessive parenchymal injury (4-7). However, there can be no substitute for patient, careful dissection.
In the intermediate volume disease there can be the temptation to remove the diaphragmatic pleura without removing the underlying muscle. One wonders whether the efforts to preserve a diaphragm that may not function well balances the risk of residual disease. Certainly, the fear that opening the pleural cavity to the peritoneal one may cause dissemination of the disease does not seem justified (8). In a few cases, full or near-full integrity of the underlying peritoneum may be technically possible however this is not easily predictable. We would suggest en-bloc phrenectomy unless there is very early disease. We would also favour diaphragmatic replacement with a bioprosthesis to reduce the risk of infective seeding of a non-absorbable material (9).
The decision to resect the pericardium is less controversial. This is associated with less morbidity and no area of doubtful excision should be left unresected. Pericardial replacement is advisable since cardiac herniation can prove to be fatal if not immediately diagnosed.
In the more locally advanced mesothelioma with lung parenchymal invasion there is the temptation to resort to EPP in the only hope of achieving macroscopic complete resection (MCR). In our experience, lobectomy, usually lower, has rarely been required and in the majority simpler wedge resection of peripheral lung invasion is all that is needed to obtain MCR.
What is the evidence for EPD over EPP?
Cao and colleagues (10) recently conducted a meta-analysis to compare the outcome of patients who underwent EPP versus the ones who underwent EPD. They identified seven studies which reported on significant comparative data between these two approaches. They concluded that EPP was associated with increased post-operative mortality and morbidity when compared with EPD.
Taioli and colleagues published a second meta-analysis which led to similar conclusions (11). EPD was associated to remarkably lower early post-operative morbidity and mortality (2.5 fold) and so should be preferred over EPP. Furthermore, the overall survival was similar after 2 years from the surgery.
The ASCO guidelines (12) stated that EPD is preferred over EPP for ‘maximal surgical reduction’ (MSR, such as resection of all macroscopic disease), but EPP may still have a role in highly selected patients and in centres with consolidated expertise. MSR is strongly recommended for early stage, but which is the best way to achieve MCR is controversial. These guidelines are in contradiction with those of the British Thoracic Society (13), which, in typically conservative fashion, portray a very limited role for definitive surgery. They suggest that EPP should be abandoned and that EPD should be offered only in the setting of a randomized clinical trial. This however may prevent some patient to receive a procedure that improves the quality of life and potentially ameliorate the respiratory function (14).
A large retrospective multi-center study (15), published in 2014, stated that ‘patients who underwent EPP had an acceptable 30 and 90 days mortality’ (4.1% and 6.9% respectively) with an overall median survival of 18.8 months. P/D reported a longer survival (20.5 months), reduced 30 and 90 days mortality (2.6% and 6% respectively), and when the analysis was restricted to the patients with positive prognostic factors the survival was still similar in the EPP and in the P/D group. Once again, a more aggressive approach, which reduces significantly the quality of life (EPP) did not seem justified.
EPD is the operation that is achieving growing acceptance worldwide (16-19). It has the aim to remove all the macroscopic disease with ‘curative’ intent. The literature cited above suggests a slightly shorter survival in patient who underwent EPP at the cost of a significantly higher operative mortality and morbidity when compared with those underwent EPD, therefore some eminent authors suggested that this procedure should be consigned to the surgical history books (20-22).
Patient selection for P/D
The current selection criteria for the MARS 2 trial (23) mandate that a MCR must be achieved by EPD. The reasons why this cannot be achieved, and therefore preclude EPD, include: multifocal chest wall invasion; invasion of major vessels i.e., aorta or subclavian on the left and the vena cava on the right; transpericardial myocardial invasion; vertebral involvement or transdiaphragmatic organ or peritoneal involvement. Nodal disease outside of the ipsilateral hemithorax (N2) is also a contraindication. Thus all T1-3, N0-1, M0 disease is suitable for EPD. It must be noted that outside of a clinical trial the presence of nodal spread preoperatively should probably be treated by induction chemotherapy.
In MARS2, patients are eligible irrespective of histology. However, the poor prognosis of sarcomatoid MPM has resulted in the ASCO guidelines (12) suggesting that major cytoreductive surgery is not indicated. One must consider, however, that there is likely to be discordance between an isolated pleural biopsy and the final histology after resection due to a potential sampling error.
Fitness for EPD should be considered to be similar to that for lobectomy for lung cancer. However, preoperative spirometric values are difficult to interpret when there is frequently pleural effusion and lung entrapment.
The two areas of debate in the selection of EPD as the operation of choice are at the two ends of the prognostic spectrum. In those with the best prognosis of epithelioid, node negative stage I disease who are young and fit should one consider the most radical treatment of extrapleural pneumonectomy? Whereas in those with bulky, node positive disease with possibly extensive lung invasion should one either consider the lesser option of VATS partial pleurectomy (V-PP) to achieve symptom control or consider EPP as the only method of achieving MCR (24,25)?
Should P/D by thoracotomy be avoided in favour of V-PP for symptom control in those with poor prognosis?
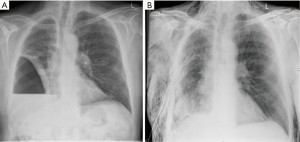
V-PP is defined as the removal, performed by video-assisted thoracoscopy, of part of the parietal and -more importantly- part of the visceral pleura as needed to achieve sufficient re-expansion of a trapped lung. When is a patient unfit for P/D and only fit for V-PP? As stated above, the physiological requirement for EPD is similar to that for a lobectomy. However, EPD should be considered in the context of multimodality therapy. Patients unfit for chemotherapy due to renal impairment may therefore not be candidates for EPD. The direction of surgery would be then only towards the palliation of symptoms, which is largely directed at effusion control. The malignant, thickened visceral pleura entraps the lung which becomes atelectatic. As a consequence, visceral pleurectomy could improve the quality of life because of the recruitment of pulmonary parenchyma (Figure 1A,B), and thus the prevention of complications arising from lung collapse and the improvement of dyspnea (25).
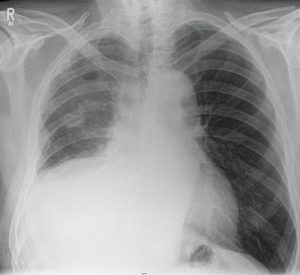
P/D could also be more effective and more tolerated, in the treatment of recurrent pleural effusion with trapped lung, when compared with indwelling pleural catheter (Figure 2). The Meso-VATS trial (26) investigated the possible role of minimally invasive surgery especially for the management of the pleural effusion and symptom control. Twelve units in UK recruited randomized patients to either talc pleurodesis or V-PP. V-PP did not demonstrate better overall survival and unsurprisingly resulted in more surgical complications and longer hospitalization than simple medical treatment. Nevertheless, there was durable improvement in the quality of life of those patients of better prognosis who underwent partial pleurectomy. Unfortunately, the patients were not stratified by the morphology of their disease. We suggest that the patients who are going to find partial pleurectomy beneficial are those with significant visceral disease and trapped lung. On the other hand, in the presence of recurrent pleural effusion but without relevant lung trapping, talc could be more than enough for symptoms control. This consideration reminds us about the heterogeneity of this disease, where every patient may be a candidate for a suitable procedure and treatment timing, in the context of a multidisciplinary, individualized approach. The British Thoracic Society does not discourage the adoption of V-PP when within a controlled clinical trial (13). The MesoTrap is a currently open trial with indwelling pleural catheter versus partial pleurectomy in patients with trapped lung (27).
What trials are available?
MARS2
There are of course intrinsic difficulties with the design and recruitment of a trial regarding a relatively uncommon disease (28-30). At the present moment, there are two ongoing trials, neither considering EPP. The MARS 2 (22) is a multi-centre UK based, phase III study which is randomizing patients to receive standard chemotherapy (platinum/pemetrexed) with or without EPD. Four surgical units (St. Bartholomew’s Hospital in London, Glenfield Hospital in Leicester, Northern General Hospital in Sheffield and Golden Jubilee National Hospital in Glasgow) are expecting to treat over 180 patients in the experimental arm referred from more than 20 medical centres. The trial is intentionally pragmatic and includes patients with non-favourable, non-epithelioid histology and node positive disease. Clearly, there is a room for potential criticism of the design. In the eventual analysis, poor survival in the operative arm may be attributed to the positive prognostic factors. However, the nature of random selection may evenly distribute these cases if the sample size is sufficiently large. It may be suspected that subgroup analysis of the benefit or harm of EPD in the best prognosis patients group may be required by many observers.
EORTC 1205
EORTC 1205 (clinicaltrials.gov) is a phase II, multicentric European trial, which is currently recruiting, and comparing upfront P/D followed by adjuvant chemotherapy with P/D after upfront chemotherapy (31). In this trial, whose results are expected in 2020, the same procedure is in both randomization arms. The trial can be criticized for assuming that P/D is the gold standard procedure, justified in all patients in advance of the conclusions of MARS2.
Of course, neither of these trials is addressing the burning question vexing many surgeons. The trial comparing the two different surgical procedures, EPP versus EPD, will have to wait until MARS3.
Should P/D be performed outside of a trial?
There is a contradiction between two recently published guidelines. Whilst the ASCO recommend cytoreductive (MCR) surgery in early stage, epithelioid MPM, with P/D preferable to EPP; the BTS state there is no role for EPP and P/D should only be offered in the context of a clinical trial (12,13).
Whilst the only route to a clearer understanding of the role of surgery in MPM is through clinical trials, like MARS2, it is very difficult to deny the possible benefits of EPD to a relatively young and fit patient with early disease who is fully informed. In these circumstances the consent process should highlight the variability in survival after EPD, the possible risks of EPD, both in terms of mortality and morbidity, and emphasize the need for multimodality treatment. The patient should be made aware of selection bias in the current published data and the current lack of unequivocal evidence.
One possible strategy is to commence a monitored program of chemotherapy as first-line treatment and then to reserve surgery for either disease progression or after accurate restaging after a fixed induction protocol of 3–4 cycles.
Conclusions
EPD is the procedure of choice in a surgical protocol for MPM. It does not carry the preclusive mortality and morbidity for the majority of the target population (males in the mid-seventies) who cannot tolerate EPP (32,33). However, EPD carries the potential benefit of MCR which lesser VATS procedures do not, and in whom no survival benefit has been demonstrated.
One can at present only postulate on what the future role for EPD will be. If MARS2 shows a survival benefit for its addition to chemotherapy then hopefully it will become more widely available. Conversely, a negative trial result will cast doubt on its efficacy but may provoke intense discussion by the international community as did the negative findings in the initial MARS trial (34,35).
One can speculate that the eventual role for EPD will be as an adjunct to systemic treatment in those of best prognosis and with lowest risk of morbidity. EPD may provide effective local control in those with the most to gain and in whom there may be a meaningful survival with the benefit of symptom control and maintained quality of life. The arrival of more effective systemic therapies will augment this strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Pass HI, Giroux D, Kennedy C, et al. Supplementary prognostic variables for pleural mesothelioma: a report from the IASLC staging committee. J Thorac Oncol 2014;9:856-64. [Crossref] [PubMed]
- Wain JC, Kaiser LR, Johnstone DW, et al. Trial of a novel synthetic sealant in preventing air leaks after lung resection. Ann Thorac Surg 2001;71:1623-8; discussion 1628-9. [Crossref] [PubMed]
- LoCicero J 3rd, Hartz RS, Frederiksen JW, et al. New applications of the laser in pulmonary surgery: hemostasis and sealing of air leaks. Ann Thorac Surg 1985;40:546-50. [Crossref] [PubMed]
- Alfano RR, Tang J, Evans JM, et al. Gelatin based on Power-gel™ as solders for Cr4+ laser tissue welding and sealing of lung air leak and fistulas in organs. Available online: https://patents.google.com/patent/US20020198517
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
- Solli P, Brandolini J, Pardolesi A, et al. Diaphragmatic and pericardial reconstruction after surgery for malignant pleural mesothelioma. J Thorac Dis 2018;10:S298-S303. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-i30. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Rodrigues AE, et al. Influence of Pleurectomy and Decortication in Health-Related Quality of Life Among Patients with Malignant Pleural Mesothelioma. World J Surg 2018;42:1036-45. [Crossref] [PubMed]
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Bertoglio P, Waller DA. The role of thoracic surgery in the management of mesothelioma: an expert opinion on the limited evidence. Expert Rev Respir Med 2016;10:663-72. [Crossref] [PubMed]
- Treasure T. Extrapleural pneumonectomy for malignant pleural mesothelioma: is this an operation that should now be consigned to history? Future Oncol 2015;11:7-10. [Crossref] [PubMed]
- Migliore M. How surgical care is changing in the technological era. Future Sci OA 2016;2. [Crossref] [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Cytoreductive surgery and hyperthermic intrapleural chemotherapy for malignant pleural diseases: preliminary experience. Future Oncol 2015;11:47-52. [Crossref] [PubMed]
- Lim E. 195 A feasibility study comparing (extended) pleurectomy decortication versus no pleurectomy decortication in the multimodality management of patients with malignant pleural mesothelioma: the MARS 2 study. Lung Cancer 2016;91:S71. [Crossref]
- Nakas A, Martin Ucar AE, Edwards JG, et al. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;33:83-8. [Crossref] [PubMed]
- Flores RM. Pleurectomy decortication for mesothelioma: The procedure of choice when possible. J Thorac Cardiovasc Surg 2016;151:310-2. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Rintoul RC. The MesoVATS trial: is there a future for video-assisted thoracoscopic surgery partial pleurectomy? Future Oncol 2015;11:15-7. [Crossref] [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- Dunning J. A view of the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial from the coalface. Eur J Cardiothorac Surg 2016;50:798-9. [Crossref] [PubMed]
- Treasure T, Dusmet M, Fiorentino F, et al. Surgery for malignant pleural mesothelioma: why we need controlled trials. Eur J Cardiothorac Surg 2014;45:591-2. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Nakas A, von Meyenfeldt E, Lau K, et al. Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;41:1031-6. [Crossref] [PubMed]
- Sharkey AJ, Tenconi S, Nakas A, et al. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632-41. [Crossref] [PubMed]
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5. [Crossref] [PubMed]


