Individual profiling of circulating tumor cell composition in patients with non-small cell lung cancer receiving platinum based treatment
Introduction
Circulating tumor cells (CTC) could serve as a “liquid biopsy” for individualizing and monitoring treatment in patients with solid tumors (1,2). So far, CTC detection methods consisted of enrichment and subsequent identification mostly with anti-cytokeratin (CK) or epithelial cell adhesion molecule (EpCAM) antibodies. CK-positive cells are thought to be absent or to be present in the blood of healthy subjects in very low numbers (3). CTC have extensively been described in breast and lung cancer and EpCAM positive CTC quantification has been linked to patient outcome (4-8). Standardized approaches with currently available enrichment and detection techniques are based on physical or biological properties of CTC and challenged by their cellular heterogeneity and plasticity. Epithelial-to-mesenchymal transition (EMT) can cause alteration of cellular features and loss of epithelial properties leading to a partial or complete switch to a mesenchymal phenotype. Particularly stem cells have the ability to take on characteristics of other cell types (9).
We recently developed a CTC detection method based on multi-immunofluorescence staining that includes but is not solely dependent on epithelial markers such as CK or EpCAM and also detects cells with mesenchymal and stem cell-like characteristics (10-12). In this study, we have addressed the question whether different types of CTC are identifiable in the peripheral blood of patients with non-small cell lung cancer (NSCLC) and, if so, whether their distribution may serve as a predictor of treatment response or outcome.
Material and methods
Informed consent and study population
Written informed consent was obtained from all patients before participating in the study. Blood sample collection and analyses were approved by the Review Board of the Medical Department, University of Essen-Duisburg; Germany (12-5047-BO). The clinico-pathological data of the patients is listed in Table 1. Tumor staging was performed according to the criteria of the International Union Against Cancer (13). Revised Response Evaluation Criteria in Solid Tumors (RECIST 1.1) were used to define response or stable disease in patients after receiving two cycles of systemic cytotoxic chemotherapy (14,15).
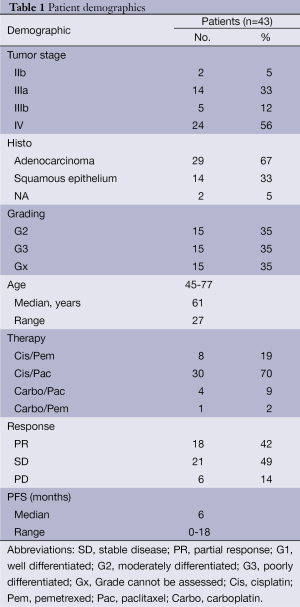
Full table
Preparation of blood samples and CTC enrichment
A total of 20 mL citrated peripheral venous blood was drawn from NSCLC patients prior to or up to three weeks after platinum-based treatment and processed within 24 h after collection. Blood sample preparation was done as follows: 20 mL of blood were diluted with 10 mL PBS and carefully layered into a Leucosep tube containing 16 mL Ficoll-Paque (GE-Healthcare) below a porous barrier. After buoyant density gradient centrifugation (1,600 ×g, 20 °C, 20 min) the interphase consisting of peripheral blood mononuclear cells (PBMNC) and CTC was removed and washed. For subtype analyses CTC were negatively enriched by hematopoietic cell depletion. PBMNC were treated with 50 µL of a 1:1 mixture of anti-CD45 and anti-CD15 coated immunomagnetic beads (Dynabeads, Invitrogen) in a magnetic particle processor (King Fisher mL; Thermo Fisher). The remaining cell suspension included bead-free pre-enriched tumor cells and was spun onto two glass slides per sample using the Cell Spin II centrifuge (Tharmac, Waldsolms, Germany), air dried and subsequently fixated with 96% Ethanol. Slides were stored at 4 °C until subjected to immunocytochemical staining.
Identification of CTC subtypes using multi-fluorescence labeling
Immunofluorescence staining of epithelial, mesenchymal, stem cell-like and hematopoietic cells was carried out in the CD45-depleted pre-enriched tumor cell suspension as described previously (10). Briefly, the staining method included fixation and permeabilization of the cells with ice-cold methanol for five min, washing in PBS, blocking of unspecific antibody reactions by incubation with blocking solution containing 5% BSA for 30 min, binding of primary antibodies (final concentration: 5 µg/mL) either pan-CK guinea pig polyclonal antibody (ABIN126062, antibodies-online) and N-cadherin (EPR1792Y) rabbit monoclonal antibody (2019-1, Epitomics) or CD133 rabbit polyclonal antibody (orb18124, biorbyt) or EpCAM (ab32392, Abcam) for CTC and anti-CD45 (MEM-28) mouse monoclonal antibody (ab8216, Abcam) for hematologic cells overnight at 4 °C, wash in 0.1% Tween, binding of secondary antibodies (FITC-conjugated AffiniPure goat anti-rabbit and Cy3-conjugated AffiniPure goat anti-mouse or AlexaFlour647-conjugated AffiniPure F(ab’)2 Fragment goat anti-guinea pig; Jackson Immuno Research, Hamburg, Germany) for 30 min at 37 °C, washing in 0.1% Tween. Subsequently, cells were stained with 4',6-Diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich, St. Louis, MO, USA) for 10 min, mounted with anti-fading medium (Invitrogen) and stored in the dark until evaluation. Microscopic evaluation was carried out using the digital Keyence BZ9000 (Biorevo, Osaka, Japan) all-in-one fluorescence microscope with integrated camera and BZ-Analyzer Software. We used pseudo colors to depict cells. Stained slides were manually examined and CTC were detected within the same areas, each consisting of ten visual fields using a 20× magnification on both slides. For CTC quantification previously published cut-offs were applied to exclude false-positive events (10).
Statistical analysis
Statistical tests were performed according to previously published studies by our group (10,16,17). The associations among CTC subtypes, and clinico-pathological parameters were tested with Spearman test for bivariate correlations. Man-Whitney test for independent samples was used to compare differences of various factors in distinct subgroups. Kaplan-Meier method was used to test correlations of PFS with cell types. Survival differences between patients with a high and low cell type ratio were analyzed by the log-rank test. The level of significance was set to P<0.05. All P values were based on two-sided tests. All statistical analyses were performed using the Software Packages JMP 10.0 Software (SAS, Cary, NC, USA), SPSS for Windows (Version 19.0; SPSS Inc., Chicago, IL, USA), and Medcalc, Version 12.3.0 (Mariakerke, Belgium).
Results
Immunofluorescence based identification of CTC subtypes
For the investigation of cellular subtypes a multi-staining method was required in order to detect various epithelial, mesenchymal, stem cell-like and hematopoietic markers and to characterize different cells types. Therefore we used multi-fluorescence staining for CTC-subtype detection in NSCLC patients. When examining samples objects that showed a positive nuclear staining with DAPI a negative staining against CD45 and a positive staining for pan-CK, N-cadherin, EpCAM or CD133 were captured and considered as tumor cells (Figure 1). In blood samples we detected cells with mesenchymal features such as N-cadherin+/CK-/CD45- and cells with epithelial properties like CK+/N-cadherin-/CD45-; CK+/EpCAM+/CD45- and cells with both characteristics like CK+/N-cadherin+/CD45-. We also detected cells showing stem cell-like features such as CD133+/CK-/CD45- and CD133+/CK+/CD45- cells. In addition, we found cells that stained positive for potential markers of CTC and CD45 such as CK+/CD45+; CK+/EpCAM+/CD45 (low); N-cadherin+/CK-/CD45 (low) and CK+/N-cadherin+/CD45 (low) as well as CK+/CD133+/CD45+ cells. We also found a sub-population of cells staining positive for CK and CD45 (low) but negative for EpCAM and cells staining triple positive for CK, EpCAM and CD45 (low). CTC were enumerated, normalized and profiles of each patient were examined. We summarized the total amount of N-cadherin-positive, CK-positive and CD133-positive cells after negative enrichment using CD45-depletion. We normalized the enumerated potential CTC against the total number of leucocytes obtained from the complete blood count and expressed the number of CTC per 1,000 PBMNC (Table 2).
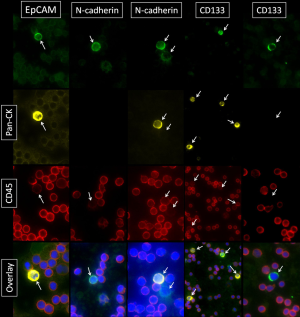
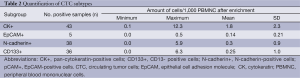
Full table
Association of CTC subtypes with clinical parameters
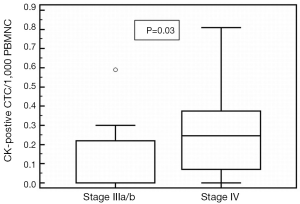
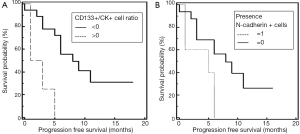
Spearman’s rank correlation revealed that the number of stem cell-like CD133-positive CTC/1,000 PBMNC (P=0.04; r=0.35) cells were significantly correlated to the amount of N-cadherin-positive CTC/1,000 PBMNC cells (P=0.002; r=0.53). Cells with epithelial characteristics (EpCAM-positive) were significantly associated to treatment response (P=0.007; r=0.97). Mann-Whitney test revealed a significant difference of CK-positive CTC/1,000 PBMNC in patients with stage IV compared to stage III NSCLC (P=0.03; Figure 2). Furthermore, Mann-Whitney test showed that CD133-positive CTC/1,000 PBMNC were significantly higher in patients with tumor grade 3 compared to grade 2 (P=0.04) and confirmed a significantly increased number of CD133-positive CTC/1,000 PBMNC in the presence of N-cadherin-positive cells (P=0.002). The number of N-cadherin-positive CTC/1,000 PBMNC was higher in patients with stage IV compared to stage III NSCLC (P=0.09) and the amount of CD133-positive cells was elevated in stage IV patients compared to stage II (P=0.1). Noteworthy, Kaplan-Meier test revealed that an increased CD133-positive to pan-CK-positive cell type ratio (stem cell like to epithelial ratio) and the presence of mesenchymal N-cadherin+ cells, both were significantly associated to shortened PFS (2 vs. 8 months, P=0.003, HR =4.43, Figure 3A; 5 vs. 8 months, P=0.03, HR =2.63, Figure 3B).
Discussion
With this study we wanted to examine the individual CTC composition in patients with NSCLC receiving platinum based treatment. Morphological analysis based on multi-immunofluorescence staining revealed a variety of CTC subtypes with epithelial, mesenchymal, stem cell-like or mixed characteristics such as CK+/N-cadherin-/CD45-; CK+/EpCAM+/CD45-; CK+/N-cadherin+/CD45-; CD133+/CK-/CD45- and CD133+/CK+/CD45- cells. Analyses of individual CTC profiles indicated that the presence of mesenchymal CTC and an increased ratio of stem cell-like to epithelial CTC was associated to poor treatment response. If CD133+ cells were detectable, N-cadherin+/CK- cells were likely to be found. Due to technical limitations (staining on two slides) it is impossible to determine whether the close association between N-Cadherin+ and CD133+ cells is related to coexpression on the same cell or to different cells. However, it seems to indicate a link between cells with mesenchymal and stem cell-like characteristics implying both as markers of poor prognosis. Cancer stem cells (CSC) and EMT-type cells are believed to play critical roles in drug resistance and cancer metastasis. The formation of CSC and the event of EMT is a dynamic process which is triggered by the interaction of various cellular signaling pathways such as Hedgehog, Notch, PDGF, Wnt, TGF-β, Akt, and NF-κB signaling pathways (18). Our results are in line with a recent study by Barr et al. who have generated an isogenic model of cisplatin resistance in a panel of NSCLC cell lines and reported that the presence and enrichment of stem-cell markers support the presence of a chemoresistant population of lung cancer cells with a stem-like signature (19). It has recently been described by Yu and colleagues that CTC undergo EMT during treatment and that these changes, and not only the absolute numbers of certain subgroups, correlate well with response and resistance to cytotoxic treatment, respectively (20).
In this study, we were able to observe a distinct proportion of cells that stained positive for pan-CK and CD45, a phenomenon already described by Yu et al. (21). The additional CD45+ staining may not be exclusive for hematopoietic cells, but may hypothetically be acquired during dormant stay in the bone marrow or through effects comparable to trogocytosis, i.e., transfer of membrane proteins (22). Though this hypothesis cannot be scrutinized by the data at hand it may warrant waiving any depletion of CD45-positive cells as this approach might lead to a loss of cells of interest. However, we took only CD133+/CD45- cells into account during the abovementioned analyses of CTC profiles with stem cell-like characteristics. Moreover, our method is based on cell type ratios rather than absolute cell numbers assuming that a loss of potential CTC might not affect the proportion of their CD45-negative subtypes. Taken together our data support the hypothesis that different CTC populations are identifiable in the peripheral blood of patients with NSCLC and that these individual cell type profiles may have distinct clinical implications. This method offers an opportunity to detect changes in the composition of circulating non-hematopoietic cells in the blood of NSCLC patients and, therefore, to create an individual profile of each patient which might help to predict patient outcome and potentially to select the appropriate treatment. Further studies addressing the question whether CTC subtype distributions are changing during platinum based treatment and whether these cells bear the potential to develop molecular markers to individualize treatment are currently ongoing.
Acknowledgements
Authors’ contributions: conception and design: Andreas-Claudius Hoffmann, Ivonne Nel; provision of study materials or patients: Andreas-Claudius Hoffmann, Thomas Gauler; collection and assembly of data: Ulrich Jehn, Thomas Gauler; data analysis and interpretation: Ivonne Nel, Ulrich Jehn, Andreas-Claudius Hoffmann; manuscript writing: Ivonne Nel, Andreas-Claudius Hoffmann; final approval of manuscript: Andreas-Claudius Hoffmann.
Funding: This project was funded by the Dr. Werner Jackstädt-Stiftung, Wuppertal, Germany. Results of this study were partly presented at the 10th Congress on Lung Cancer of the Spanish Lung Cancer Group in Barcelona, Spain in November 2013.
Disclosure: The authors declare no conflict of interest.
References
- Lianidou ES, Markou A. Circulating tumor cells as emerging tumor biomarkers in breast cancer. Clin Chem Lab Med 2011;49:1579-90. [PubMed]
- O’Flaherty JD, Gray S, Richard D, et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer 2012;76:19-25. [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [PubMed]
- Moldenhauer G, Momburg F, Möller P, et al. Epithelium-specific surface glycoprotein of Mr 34,000 is a widely distributed human carcinoma marker. Br J Cancer 1987;56:714-21. [PubMed]
- Zhang N, Li X, Wu CW, et al. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene 2013;32:5078-88. [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [PubMed]
- Gauler TC, Theegarten D, Parr A, et al. Decrease of circulating tumor cells associates with response to platinum-based chemotherapy in patients with non-small cell lung cancer, but not with small cell lung cancer. J Thorac Oncol 2011;6:S1114.
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [PubMed]
- Nel I, Baba HA, Ertle J, et al. Individual profiling of circulating tumor cell composition and therapeutic outcome in patients with hepatocellular carcinoma. Transl Oncol 2013;6:420-8. [PubMed]
- Nel I, Gauler TC, Eberhardt WE, et al. Formation and repair kinetics of Pt-(GpG) DNA adducts in extracted circulating tumour cells and response to platinum treatment. Br J Cancer 2013;109:1223-9. [PubMed]
- Nel I, Gauler T, Hoffmann AC. Circulating tumor cell composition and outcome in patients with solid tumors. Int J Clin Pharmacol Ther 2014;52:74-5. [PubMed]
- Sobin LH. TNM: evolution and relation to other prognostic factors. Semin Surg Oncol 2003;21:3-7. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Hoffmann AC, Wild P, Leicht C, et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010;12:628-36. [PubMed]
- Hoffmann AC, Danenberg KD, Taubert H, et al. A three-gene signature for outcome in soft tissue sarcoma. Clin Cancer Res 2009;15:5191-8. [PubMed]
- Sarkar FH, Li Y, Wang Z, et al. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir 2009;64:489-500. [PubMed]
- Barr MP, Gray SG, Hoffmann AC, et al. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS One 2013;8:e54193. [PubMed]
- Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-4. [PubMed]
- Yu M, Stott S, Toner M, et al. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 2011;192:373-82. [PubMed]
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 2003;4:815. [PubMed]


