
Medically inoperable stage I non-small cell lung cancer: best practices and long-term outcomes
Lung cancer remains the leading cause of cancer death in the U.S. and worldwide (1,2). According to projected estimates for 2018, more than 234,000 Americans will receive a new diagnosis of lung cancer, and over 154,000 will succumb to their disease (3). Non-small cell lung cancer (NSCLC) represents the majority of new lung cancer diagnoses, accounting for over 85% of cases, with about 16% of these patients presenting with early stage disease, as defined by disease limited to the chest and with primary tumor size of less than 5 cm (4).
Of note, the recent update in the AJCC staging system has affected this group of patients: previously classified as T1 (≤3 cm) or T2a (>3 to ≤5 cm), N0, M0, stage IA–IB, per 7th edition staging, these tumors are now defined in the 8th edition as T1 (≤3 cm), T2a (>3 to ≤4 cm), or T2b (>4 to ≤5 cm), N0, M0, stage IA–IIA (Table 1) (5,6). As the majority of modern SBRT trials and data to date utilize the AJCC 6th, or more commonly, 7th, edition staging, references to disease stage will remain consistent and also use this nomenclature unless otherwise specified (6,7).

Full table
The minority of lung cancer patients have early stage disease at presentation, but with increases in general medical imaging and the growing adoption of low-dose computed tomography (CT)-screening protocols for those identified as high risk for developing lung cancer (age 55–74 years and ≥30 pack-year history of smoking and <15 years since smoking cessation, or age ≥50 years and ≥20 pack-year smoking history and additional risk factors that increase risk of lung cancer to ≥1.3%, i.e., personal history of cancer or lung disease, family history of lung cancer, radon exposure, occupational exposure to carcinogens, not including second hand smoke) (8-11), this group is and will likely continue to become an increasingly prevalent proportion of lung cancer patients (12). Additionally, with continued improvement in treatment algorithms, more individualized therapies based on patient and tumor characteristics, and treatment morbidity reduction techniques, this group is becoming a population with more potential for long-term disease control and survival, a change likely contributing in part to the annually decreasing death rate for NSCLC by 3.8% for men and by 2.3% for women since 2011 (13). Therefore, continued optimization of the management of these patients is critical.
Work-up
In general, new lung masses are (I) discovered on work-up of local symptoms such as cough, hemoptysis, weight loss, or chest wall pain; (II) incidentally noted on imaging for a different medical issue; or (III) detected on low-dose screening chest CT scan for high risk patients. Patients with early stage disease are more likely to have disease identified in the latter two scenarios as the presence of pulmonary or systemic symptoms generally indicates more advanced disease at presentation.
On discovery of a suspicious lung mass, definitive imaging studies should be obtained if not already performed, including a diagnostic or high-resolution CT scan of the chest. If the mass is found to possess characteristics concerning for malignancy, such as spiculated or irregular borders, lack of benign-appearing calcifications, or significant solid component, an attempt to obtain tissue from the mass should be made to establish a histologic diagnosis (Figure 1). This can be performed in several ways and is dependent on multiple factors including the size and location of the tumor, patient characteristics (comorbidities), and local expertise. The most common biopsy approaches for lung masses include peripheral CT-guided biopsy, transbronchial biopsy, endobronchial ultrasound (EBUS), navigational bronchoscopy, or transthoracic needle aspiration. Final determination of the optimal technique is left to the discretion of the proceduralist, frequently a pulmonologist, interventional radiologist or thoracic surgeon.
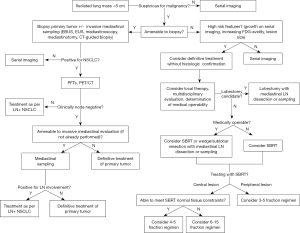
For a subset of patients, the standard diagnostic approach of histologic confirmation via biopsy is not possible due to (I) unacceptably high risk of complication with biopsy due to patient’s comorbid status, (II) non-diagnostic tissue obtained on biopsy, or (III) patient refusal. Discussion with multidisciplinary participation should take place to determine the best approach for their management. Patients with a prohibitively high risk of morbidity with biopsy are often not candidates for surgical resection; similarly, those who refuse biopsy often also have a strong preference to avoid definitive surgery. For these patients, multiple prospective and retrospective studies assessing the role of definitive SBRT for early-stage NSCLC (ES-NSCLC) have included patients without a biopsy-proven diagnosis and have found no difference in local, regional, or distant disease control, or in survival outcomes (14-17). Importantly, these studies stipulated that these tumors were considered to have a high likelihood to represent malignancy based on features suggestive of malignancy such as lesion size, tumor growth on serial CT scans, or increased FDG uptake on FDG-PET/CT, based on thorough evaluation including multidisciplinary tumor board review. Predictive models are also available, with which the probability of malignancy can be calculated based on clinical and radiographic characteristics (18,19).
After a diagnosis of NSCLC has been made, staging workup, baseline pulmonary function tests (PFTs) and smoking cessation counseling when applicable, are indicated. Nodal evaluation with [18F]-fluoro-2-deoxy-glucose positron emission tomography with computed tomography (FDG-PET/CT) and invasive mediastinal staging are generally indicated to rule out occult mediastinal nodal involvement not detected on CT imaging alone, which has a sensitivity of roughly 60% and specificity of 80% for detection of mediastinal nodal disease (20). Historically, invasive mediastinal nodal evaluation has been the accepted gold standard for mediastinal staging, which can be accomplished with endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), EBUS-guided transbronchial needle aspiration (EBUS-TBNA), navigational bronchoscopy, or mediastinoscopy. The advantages of direct lymph node station sampling by these methods include more definitive assessment of the presence of nodal spread of disease and the ability to obtain additional molecular and histologic information that may guide systemic therapy in the case that more advanced disease is uncovered.
In recent years, FDG-PET/CT has become a critical adjunct staging study for a growing number of disease sites, such as B-cell lymphoma, anal carcinoma, and cervical malignancies. FDG-PET plays a role of particular importance in mediastinal lymph node assessment for NSCLC, for which it has a sensitivity of 79–85% and specificity of 87–92% (21-23). In fact, FDG-PET/CT may detect occult hilar and mediastinal lymph node involvement with comparable accuracy to that of more invasive lymph node staging. A meta-analysis by Wang et al. determined that the negative predictive value (NPV) of FDG-PET/CT was high, up to 94% for tumors ≤3 cm and 89% in tumors >3 cm (20). This is similar to the sensitivity and NPV of 79–81% and 93%, respectively, achieved with invasive mediastinal staging via EBUS/EUS or mediastinoscopy (24). In a recent multicenter analysis of 233 patients with ES-NSCLC who received definitive treatment with SBRT, pre-treatment staging with invasive mediastinal lymph node staging in addition to PET/CT (180 patients, 199 lesions) did not result in an increase in regional failures compared with PET/CT staging alone (56 patients, 58 lesions) (11% PET/CT vs. 21% PET + mediastinal staging, P>0.05) (25). Therefore, mediastinal staging without the use of invasive procedures may be a reasonable approach for regional lymph node assessment. This is significant for patients who are not optimal surgical candidates, for whom FDG-PET/CT alone can be used for staging without significantly impacting the ability to perform a robust staging workup.
For patients who are borderline candidates for surgery, the omission of invasive mediastinal staging may be permissible if certain criteria suggestive of a lower probability of harboring occult mediastinal disease are met. Examples of these disease characteristics include peripheral tumor (outer 1/3 of the lung), tumor diameter of ≤3 cm, and absence of suspicious intrathoracic lymph nodes on the CT portion of FDG-PET/CT (26). Conversely, patients considered at increased risk of undetected nodal involvement such as those with tumors greater than 2 cm in size, central tumor location, or FDG-avid tumors on FDG-PET/CT, should still strongly consider undergoing invasive mediastinal staging to avoid undertreatment of clinically occult stage II or III disease (27). A balanced discussion of the potential risks and benefits of each approach to invasive mediastinal staging should take place between the patient and provider so that an informed decision can be made.
Determination of medical inoperability
The standard treatment approach for ES-NSCLC in medically operable patients is surgical resection with lobectomy and mediastinal lymph node sampling. However, surgery is sometimes not a viable option in this patient population. With an average age of diagnosis of 70 years, lung cancer patients often have a level of baseline frailty, along with concomitant comorbid conditions, especially those associated with risk factors for NSCLC such as heart disease, chronic obstructive pulmonary disease (COPD), and loss of pulmonary parenchyma (28). Combining these factors, around 25% of patients with ES-NSCLC lack the medical fitness necessary to undergo definitive surgical resection (29). In addition, some patients prefer not to undergo an operation and desire noninvasive treatment.
The determination of medical operability for ES-NSCLC is frequently made using the ability to undergo a standard oncologic resection, i.e., lobectomy, as the baseline metric. A number of factors such as performance status, presence of medical comorbidities, and pulmonary function tests (PFTs), contribute to overall risk assessment. While there is not one globally accepted definition of medical operability, one common classification schema separates patients into high or standard operative risk groups. High operative risk patients are considered those who cannot tolerate a lobectomy but who may be candidates for a more limited surgery such as sublobar or wedge resection and could also be considered for definitive radiation therapy with stereotactic body radiation therapy (SBRT). Standard operative risk patients are thought to have an anticipated operative mortality of <1.5% and can likely undergo a lobectomy with limited likelihood of perioperative morbidity.
While this dichotomous division may appear simple, categorizing patients into one of these two groups is often not a straightforward distinction. Pulmonary function measures are used to help determine operability, with each assigned risk levels based on numeric limits. Most commonly applied PFT criteria include FEV in 1 second (FEV-1) and median diffusion capacity to carbon monoxide (DLCO). In RTOG 0236, eligible patients were deemed medically inoperable if they fulfilled one of: FEV-1 <40% predicted, predicted postoperative FEV-1 <30% predicted, DLCO <40% predicted, baseline hypoxemia or hypercapnia, severe pulmonary hypertension, diabetes mellitus with end organ damage, severe cerebral, cardiovascular, or peripheral vascular disease, or severe chronic heart disease (30). These criteria are similar to those still used for patient selection in more recent trials (31). Due to the unique nature of each patient’s comorbid makeup, along with the variability of the thoracic surgery team, individual surgeon, and institutional experience with different degrees of preoperative risk, patients should be approached on an individualized basis with input from the multidisciplinary team and final determination made by the treating thoracic surgeon.
The degree of fitness needed to undergo SBRT without significantly compromising lung function from treatment-related pulmonary insult, particularly in the common presence of underlying lung disease, has been called into question. Medically inoperable patients enrolled on RTOG 0236 were assessed for changes in PFTs and arterial blood gas changes after SBRT to identify any correlates between SBRT and treatment morbidity. After 2 years of follow-up, small declines in arterial blood gases and oxygen saturation were noted, while changes in mean FEV-1 and DLCO of 5.8% and 6.3%, respectively, were found. These changes did not translate to clinically significant adverse effects. In addition, baseline PFTs were not predictive of toxicity or survival decrements, nor were lung dosimetric endpoints such as lung V5, V10, V20, or mean lung dose (32). A number of reviews have also corroborated these findings, and many practitioners today do not use baseline PFTs as an absolute contraindication in their decision to treat ES-NSCLC with SBRT (33,34). Care should be taken to minimize the dose delivered to normal lung tissue in these patients with compromised lung function, as with all patients receiving radiation therapy.
The pre-existence of interstitial lung disease (ILD), however, may increase the risk for symptomatic and severe radiation pneumonitis after SBRT (35,36). Ueki and colleagues reported on a series of 157 patients who received SBRT for ES-NSCLC, of whom 20 had baseline ILD (36). Pre-existing ILD was predictive of grade ≥2 (55.0% versus 13.3%, P<0.001) and grade ≥3 (10.0% versus 1.5%, P=0.020) radiation pneumonitis. Pre-existing ILD and volume of irradiated lung were also found to be risk factors for radiation pneumonitis on multivariate analysis, with a trend for worse overall survival for patients with ILD (53.8% versus 70.9%, P=0.28). In these patients, greater caution to minimize the volume of irradiated lung should be taken until additional data about this population are obtained.
SBRT dose-fractionation
Prior to the introduction of the extreme hypofractionation of SBRT in the lung cancer treatment armamentarium, local radiotherapy to small, early stage tumors was approached similarly to more advanced disease and treated to a dose of 60 to 70 Gy. This dose and associated biological effective dose (BED) achieved local control rates of 30–93.7% and 3- and 5-year overall survival and cause-specific survival rates of 34% and 21%, and 39% and 25%, respectively (37). By using SBRT, a higher BED can be delivered to these tumors, with a threshold BED of ≥100 Gy shown to result in an improved rate of local control and overall survival (38). Overall survival is generally also found to be superior to treatment with conventional fractionated radiotherapy, but as this is often a selected population with a baseline limited life expectancy due to comorbidities precluding surgical treatment, this endpoint has not seen improvements to the same degree as local tumor control endpoints (39,40).
The Stereotactic Precision and Conventional radiotherapy Evaluation (SPACE) trial, which randomized 102 patients with medically inoperable stage I NSCLC to SBRT (66 Gy in 3 fractions) or conventional fractionated radiation therapy (70 Gy in 35 fractions), was recently reported (41). Although numerically improved, local control rates were not significantly superior with SBRT versus 3D-conformal radiotherapy (3DCRT) (70% vs. 59%, P=0.26), although more T2 tumors (P=0.02) and male patients (P=0.35) were included in the SBRT arm, known poor prognostic factors. Survival endpoints were also not different, although toxicity and quality of life outcomes were found to be better with SBRT. Therefore, as a more convenient and cost-effective treatment option with a superior toxicity profile versus conventional fractionated radiotherapy and likely superior disease control given all available to date, SBRT is still the optimal approach to definitive radiotherapy in appropriate candidates with ES-NSCLC.
The principle utility of SBRT is in its ability to treat small lesions by delivering large doses in relatively few fractions. SBRT courses in the U.S. are considered those delivered in 5 or fewer fractions, although courses of up to 10 to 15 fractions are often also included in the discussion of lung SBRT as reasonable alternatives for some cases, as will be discussed. SBRT is made possible by the rapid dose fall-off beyond the tumor volume, which is achieved using multiple highly conformal coplanar and non-coplanar photon beams (42). Tumor volume is a limiting factor in the use of SBRT and should generally remain around 5 cm or smaller in the lung to maintain acceptable PTV coverage, although recent reports of SBRT for tumors >5 cm demonstrate promising results (43,44). The maximal avoidance of adjacent normal tissue is particularly significant in lung malignancies, as sensitive thoracic structures often lie near or adjacent to the tumor.
Critical in approaching a patient with early stage lung cancer being considered for SBRT is tumor location in the lung, traditionally a distinction of central or peripheral as first conceptualized by Timmerman et al. after an excess of grade 3 or worse adverse events was observed in a phase II study from Indiana University in which 70 patients were treated with 60–66 Gy delivered over 3 fractions for medically inoperable stage I NSCLC (Figure 1) (45). Six likely treatment-related deaths from fatal hemoptysis, infectious pneumonia, and pericardial effusion were observed, and on further analysis, central tumor location was identified as a predictor of severe toxicity, with the number of grade 3–5 adverse events observed in patients with central versus peripheral tumors increased almost threefold (27.3% versus 10.5%, P=0.088). Therefore, eligible patients in the subsequent RTOG 0236 were required to have tumors that were at least 2 cm in all directions from the proximal bronchial tree, defined as the distal 2 cm of the trachea, carina, and named major lobar bronchi up to their first bifurcation and, therefore, were not prohibitively “central” in location (Figure 2) (30). The definition of central has since been expanded in recent trials to include tumors abutting the mediastinum, pericardium, and spine (46), while that of non-central, or peripheral, tumor location, now sometimes stipulates a distance of at least 2 cm from the esophagus, heart, spinal cord, great vessels, phrenic nerve, and recurrent laryngeal nerve, in addition to those structures initially specified (47).
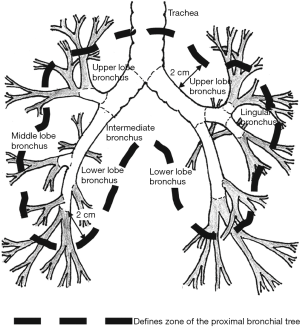
Of note, RTOG 0236 was not only a landmark study for SBRT as the first North American prospective cooperative group trial evaluating this modality for ES-NSCLC but was also fundamental in laying the groundwork for the adoption of standards in SBRT data evaluation and patient selection. With the toxicity seen in patients with central lesions, the critical significance of tumor proximity to the central structures was recognized and has been validated on numerous trials since its initial reporting. In addition, the definitions of failure used in their study are still considered standard, with recurrence after SBRT classified as in-field, in-lobe, regional, or distant.
Central tumors
Given results from the aforementioned study from Indiana University, a three-fraction regimen should be avoided for central lesions, as damage to adjacent mediastinal structures can be severe and even fatal. Potential complications include fatal hemoptysis, tracheal/airway perforation or fistula, great vessel rupture, spinal cord myelopathy, esophageal ulceration or fistula, and fatal airway necrosis. This approach has been validated in multiple other retrospective series in which central tumors treated with hypofractionated regimens have resulted in significant morbidity (48-51).
Efforts are ongoing to identify an SBRT regimen with a more acceptable toxicity profile that decreases the cumulative BED delivered to critical central structures. Attempts have been made to find a balance between increasing the number of fractions delivered to 4 or 5 and simultaneously decreasing the dose delivered per fraction, while maintaining the cumulative BED at a level needed to achieve good local control.
Results with this approach have been mixed in a number of retrospective series reported to date. At MD Anderson Cancer Center (MDACC), 27 patients with primary or recurrent ES-NSCLC measuring ≤4 cm were treated first with 40 Gy in 4 fractions (52). As no serious adverse toxicities occurred after 3 months of follow-up, the dose was subsequently increased to 50 Gy in 4 fractions. Of the patients treated with 50 Gy, the crude local control rate was 100% after 17 months median follow-up. Toxicity was relatively limited, with 3 patients (11.1%) developing grade 2–3 dermatitis and chest wall pain, and one patient developing brachial plexopathy after receiving a dose of 40 Gy to a significant volume of the brachial plexus.
Rowe and colleagues at Yale University also reported on their series of 47 patients with 51 centrally-located primary or metastatic lung lesions treated with 3- to 5-fraction SBRT, with the majority similarly receiving 50 Gy in 4 fractions (57%), and 75% of patients receiving a cumulative BED of at least 100 Gy (53). Actuarial 2-year lobar local control was 94%, although for those who received a BED of ≥100 Gy, local control at two years was 100%, whereas patients treated with a regimen delivering BED <100 Gy experienced a local control of only 80% (P=0.02). Four grade 3 events were reported in the form of dyspnea, while no grade 4 toxicity occurred. One patient developed likely treatment-related grade 5 hemoptysis.
In a phase II prospective trial from Belgium, 17 patients with centrally-located NSCLC ≤6 cm in size (median tumor diameter 3 cm) were treated with 60 Gy in 4 fractions of SBRT, while 23 patients with peripherally-located NSCLC were treated with 60 Gy in 3 fractions (51). After a median follow-up of 16 months, among patients with central tumors, local tumor control was excellent, with only 1 local failure noted. The degree of toxicity observed appeared to be dependent on tumor location and PTV size for this group, with one patient dying from bleeding after bronchial stent placement for bronchial stenosis that developed after SBRT.
These and other reports of SBRT for central tumors are limited in their use of a variety of dose-fractionation schema, short follow-up data, and non-uniform reporting of clinical endpoints; more systematic investigation on prospective clinical trials is needed to address the question. RTOG 0813 was a phase I/II study that employed a tiered approach to dosing and included 100 medically inoperable patients with centrally located stage I NSCLC measuring less than 5 cm (46). Radiation dose was delivered in 5 fractions given every other day, from a total dose of 50 Gy up to 60 Gy, increasing in increments of 0.5 Gy per fraction from 10 to 12 Gy. Dose-limiting toxicity was defined as grade 3 or worse adverse events occurring within 12 months after SBRT, with additional events beyond that time window still being recorded. Early results at a median follow-up of 26.6 months showed that while there were no DLTs reported for the 10 Gy per fraction arm, 4 fatal grade 5 toxicities have occurred thus far, all in the form of hemoptysis (1 in the 10.5 Gy per fraction arm within 12 months, 2 in the 11 Gy arm after 12 months, 1 in the 12 Gy arm after 12 months). An additional 10 Grade 3 events and 1 grade 4 events were observed in the groups receiving greater than 10 Gy per fraction.
It is clear that the risk for serious complications remains an issue for central structures with even small incremental increases in doses for 4- to 5-fraction regimens. For patients with tumors intimately juxtaposing critical structures, termed ultra-central tumors, more mild hypofractionation using 6 to 15 fractions may be indicated in an attempt to limit risk and harm to patients. Prospective and retrospective investigations have demonstrated excellent local control and acceptable toxicity rates at 3 years using 60 Gy in 8 fractions and 70 Gy in 10 fractions for central ES-NSCLC (54-56).
As more information is gathered on the optimal dose regimen for tumors adjacent to the proximal bronchi, goal dose constraints from current prospective protocols such as RTOG 0813 can be used for treatment planning. For example, although technically still experimental, criteria such as limiting the volume of proximal bronchial receiving >18 Gy to <4 cc and limiting a maximum point dose to 105% of the PTV prescription dose can give guidance for planning when using a 5 fraction SBRT for central tumors.
Other mediastinal structures at risk for serious complication when intimately involved by or abutting tumor include the esophagus, heart, pericardium, and great vessels. In particular, the esophagus can pose a significant issue when rare but potentially life-threatening high-grade toxicities occur, such as with stricture, perforation, tracheoesophageal fistula, and ulceration. Lower grade adverse events such as dysphagia, odynophagia, weight loss, and nausea can usually be managed with conservative measures. As with many structures, dose tolerances for the esophagus in the setting of SBRT are not well-defined; however, given that the esophagus is a serially-functioning organ and the volume of esophagus in the high-dose area is limited for ES-NSCLC, maximal point doses and small-volume dose constraints are likely most relevant in assessing esophageal dose for these plans. Wu and colleagues at Memorial Sloan-Kettering Cancer Center evaluated dose-volume histograms of 125 patients treated with SBRT for central lung tumors and determined correlations with significant dose constraints on logistical modeling (57). D5cc and Dmax were predictors of toxicity, and to achieve a ≤20% grade ≥2 acute toxicity rate, a D5cc of less than 16.8 Gy, 18.1 Gy, and 19.0 Gy and Dmax of less than 27.6 Gy, 30.2 Gy, and 32.2 Gy were needed for SBRT with 3-, 4-, and 5-fraction regimens, respectively. Other examples of dose-volume constraints that have been suggested for 5-fraction SBRT include Dmax <50 Gy and D1cc <45 Gy (58).
SBRT for central tumors located near the heart, large vessels, and pericardium can cause infrequently reported but severe morbidity. Reports of pulmonary hemorrhage (albeit in the setting of vascular endothelial growth factor therapy), pericardial effusion, pericarditis, myocardial infarction, and bradycardia, have been cited in retrospective and prospective studies evaluating SBRT delivered to central ES-NSCLC tumors in doses ranging from 35–60 in 3–5 fractions (46,48,51,59). Optimal dose-volume limits for these structures in the setting of SBRT have yet to be determined. In RTOG 0236, the heart was limited to a maximum point dose of <30 Gy for a 3-fraction SBRT regimen delivering a total of 54 Gy, with no serious cardiac toxicity reported (30). In the recent RTOG 0813 protocol in which 50 to 60 Gy was delivered in 5 fractions, the heart and pericardium were limited to <15 cc receiving up to 32 Gy, or 6.4 Gy per fraction, and non-adjacent walls of the great vessels receiving up to 47 Gy, or 9.4 Gy per fraction; no grade 3 or worse cardiac events have been observed to date using these parameters (46).
Data available to date highlight the need for further study to identify dose-fractionation regimens that marry the twin goals of delivering sufficient tumoral BED while maintaining an acceptable safety profile. Until these are established, dose limits delineated in prospective trials, such as those found in RTOG 0813, should be utilized to help guide radiation planning (31). Careful patient and dose selection based on tumor size, location, and comorbidities should be taken into consideration, as should the individual patient’s preference after a thorough discussion of the risks and benefits of the different available treatment options occurs.
Peripheral tumors
Peripherally-located ES-NSCLC presents disparate and generally is associated with more limited complications compared with the challenges introduced by central tumors. Adverse events associated with peripheral lesions near or abutting the chest wall include soft tissue/nerve pain such as chest wall pain syndrome and rib fracture. After recognition of these potential side effects with SBRT, this toxicity endpoint has been more consistently reported. In general, chest wall pain associated with SBRT is not severe, most commonly grade 1 or 2, occurring in 5–45% of patients with tumors abutting the chest wall (30,60). In these and for uncommon occurrences of grade 3 chest wall pain, management remains conservative with narcotic or non-narcotic analgesics and/or anti-inflammatory medications. Rare reportings of treatment-related rib fracture and more severe chest wall pain that are not self-limited and instead persists for a prolonged period of time have been cited in retrospective series (61).
Because of the frequency and at times moderate to severe nature of chest wall-related events, efforts have been made to identify dose parameters and risk factors that predict for an increased likelihood of chest wall toxicity. Several associations have been identified that may be associated with a higher rate of toxicity, including >30–35 cc of chest wall receiving 30 Gy, >3 cc of chest wall receiving >60 Gy, and use of three-fraction regimens versus more fractions (61-65).
As the majority of events can be readily controlled with minimal intervention and as even higher grade events do not pose a life-threatening consequence, the need for planning modifications with PTV coverage compromise or other more conformal techniques generally is not indicated. Continued study in identifying potential dose constraints that can be readily applied for peripheral tumors to minimize the risk of serious chest wall complications is needed.
Long term outcomes of clinical trials
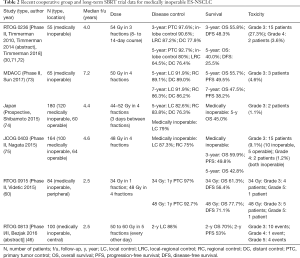
To date, acceptance of SBRT as a standard treatment modality for ES-NSCLC has been hindered in large part due to the lack of mature outcomes data available in trials studying this relatively young technology. Early evidence has provided a foundation in support of the use of definitive SBRT in patients who are not candidates for lobectomy, demonstrating 3-year local failure rates of 4–11%, comparable to those seen after sublobar resection. Overall survival in this population varies greatly across studies and is generally lower than that of patients who receive definitive surgery due to patient selection and pre-existing comorbid status, as well as differences in clinical versus surgical staging (30,66-70). However, hesitation still persists due to an as yet limited understanding of the long-term equipoise between surgical resection and SBRT in terms of local control, regional control, survival, and toxicity. In the last several years, an increasing number of trials have reported more mature outcomes for patients with inoperable ES-NSCLC, with promising results (Table 2).
Full table
The seminal phase II trial, Radiation Therapy Oncology Group (RTOG) 0236, evaluated 55 medically inoperable patients with peripheral, biopsy-proven NSCLC ≤5 cm in diameter who were treated with 54 Gy in 3 fractions delivered over 8–14 days. Initial reporting of 3-year outcomes demonstrated an excellent local control rate of 97.6% and overall survival of 55.8% (30). Five-year data has recently been reported, revealing that primary tumor control remained excellent at 92.7%, consistent with the <10% local failure rate achieved in similar studies (71,72). In-lobe failure and distant failure did increase with time, with recurrence rates of primary tumor and involved lobe recurrence of 20%, regional recurrence of 10.9%, and distant recurrence of 23.6%. Overall survival at 5 years was 40%. At 5 years, there were still no grade 5 adverse events, and the incidence of grade 3 or 4 toxicities remained low, with 15 patients (27.3%) developing a grade 3 toxicity and 2 patients (3.6%) experiencing a grade 4 toxicity (71,72). Only two additional patients developed a serious adverse event between 3- and 5-year analyses, suggesting that longer term follow-up of similar investigations will not reveal a significant unfavorable shift in what has to date been the excellent toxicity profile of SBRT.
Investigators at MDACC recently reported 7-year outcomes of a phase II trial in which 65 patients with medically inoperable clinical stage I NSCLC patients were treated with SBRT to a dose of 50 Gy in 4 fractions. All patients had histological confirmation of disease and were staged by FDG-PET/CT. Local control, regional control, and distant control were achieved in 91.9%, 89.1%, and 89.0% of patients at 5 years and in 91.9%, 86.3%, and 86.2% of patients at 7 years, respectively. Five- and 7-year progression-free survival were 49.5% and 38.2%, respectively, and 5- and 7-year overall survival were 55.7% and 47.5%, respectively. Toxicity was minimal, with only 3 patients experiencing grade 3 treatment-related adverse events (73). This is the longest follow-up data in a prospective SBRT trial reported to date and demonstrates the persistence of excellent long-term tumor control.
In a Japanese prospective study, 180 patients (120 medically inoperable, 60 operable) with stage I NSCLC were treated with SBRT with one of three dose regimens based on tumor size; 44–52 Gy were delivered in 4 fractions, with at least 3 days required between each fraction (74). Five-year outcomes are available for this trial, with an overall survival rate of 52.2% and local, regional and distant control rates of 82.6%, 83.8%, and 87.3%, respectively. On analysis of only medically inoperable patients, local control was 79%. Only 2 patients (1.1%) developed grade 3 toxicities, and no grade 4 or 5 adverse events were reported. These findings are in keeping with those reported in the aforementioned MDACC study that used a similar fractionation regimen.
The Japan Clinical Oncology Group (JCOG) 0403 phase II trial evaluated 164 patients (100 inoperable, 64 operable) with histologically or cytologically proven clinical T1 NSCLC treated with SBRT to 48 Gy in 4 fractions (75). At the most recent reporting of 3-year endpoints, similar findings were seen, with overall survival for inoperable patients of 59.9% and local control of 87.3%. Regional failures developed in 25% of patients. Grade 3 toxicities was observed in 15 patients (10 inoperable, 5 operable), and 2 grade 4 toxicities were observed (both inoperable). No grade 5 toxicities were reported.
Initial results have been published for two more recent trials. RTOG 0915 was a phase II trial comparing two SBRT dose-fractionations in 84 medically inoperable patients with peripheral stage I NSCLC (60). Patients were randomized to receive either 34 Gy in 1 fraction or 48 Gy in 4 consecutive daily fractions. After a median follow-up of 30.2 months, the 2-year overall survival, 2-year disease-free survival, and 1-year primary tumor control were 61.3%, 56.4%, and 97% in the 34 Gy arm, respectively, and 77.7%, 71.1%, and 92.7% in the 48 Gy arm, respectively. Both arms met pre-specified grade ≥3 adverse event goals, and of note, no grade ≥3 chest wall toxicities were seen even in patients receiving the single fraction regimen.
Optimal dose-fractionation regimens are also still under investigation for centrally located tumors, and long-term toxicity and efficacy data of RTOG 0813 are eager awaited.
Although reports to date are promising for the continued efficacy of SBRT for ES-NSCLC on long-term follow-up, the significance of lengthy surveillance is illustrated in a retrospective review of 66 patients treated with SBRT with histologic confirmation with 48 Gy in 4 fractions (76). While the 5-year overall survival of 44.6% was comparable to that seen in similar studies to date, 2 of 16 patients who survived without disease progression at 5 years developed late local recurrences over 76 months after SBRT.
As more lengthy follow-up data are gathered and reported, the role of SBRT in medically inoperable patients will become increasingly clear. This information, in combination with results from ongoing trials directly comparing SBRT and surgery for operable patients, should provide the evidence needed in the community to embrace SBRT as a definitive treatment option for ES-NSCLC as a way to potentially impart equivalent disease control with superior safety versus traditional surgical approaches.
Surveillance
Post-treatment surveillance protocols for SBRT have not been firmly established, although close monitoring after treatment is essential. Assessment of tumor control after SBRT can pose a significant challenge due to the development of post-treatment changes on follow-up imaging. These difficulties in interpretation of surveillance images can lead to missed diagnoses or unnecessary additional testing in the form of imaging or biopsy. On CT, post-radiation benign inflammation, fibrosis, and atelectasis can be difficult to distinguish from residual or recurrent tumor. FDG-PET/CT evaluation can also be confounded by persistent SUV elevations from post-treatment inflammatory and fibrotic changes that can last for 2 or more years, leading to a high false positive rate (77-79).
While the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (80) provide a standardized approach to identification of tumor characteristics concerning for progression, in the case of post-SBRT local tumor surveillance, the need for a more formal scoring system taking into account the unique imaging changes that can develop after SBRT has been suggested (81). These include tumor characteristics such as adjuvant structure infiltration, bulging margins, growth in a mass-like, spherical, craniocaudal, or sustained pattern, loss of air bronchograms, enlargement after 1–2 months, and linear margin disappearance (81,82).
Several guidelines have been developed in recent years to establish a more standardized approach to post-SBRT surveillance. Results from an International Delphi Consensus Study recommend post-treatment CT imaging at months 3, 6, and 12 in year 1, months 18 and 24 in year 2, and annually thereafter up to 5 years. Closer surveillance in the first 2 years following SBRT is of particular importance given that the highest rate of local recurrence is seen in this time period (81,83,84). Imaging at 6 weeks post-treatment was considered not necessary, while frequency of imaging after 5 years was recommended but at an undefined frequency. The panel also felt that FDG-PET/CT should be obtained only in the event that a surveillance CT scan was suspicious for local recurrence. Other investigators have recommended that FDG-PET/CT should not be obtained for at least 6 months after SBRT due to the high likelihood for elevated FDG uptake from either slow metabolic resolution of tumor or post-treatment changes (85), whereas others have advocated for its use during this early time interval for prognostication (86).
Salvage treatment options after recurrence include repeat SBRT, salvage surgery or chemoradiation (87), although for those patients who are medically inoperable at the time of SBRT either due to personal preference or due to medical limitations, salvage surgery will likely not be an option in the setting of recurrence. For this group, repeat SBRT after primary SBRT may be a viable option and can result in excellent control of local and regional recurrence. In a recent reporting of long term results from a phase II study of 65 medically inoperable patients with stage I NSCLC treated with, 13 patients developed locoregional recurrence (local recurrence in 5 patients, regional recurrence in 8 patients) (73). Of patients who underwent salvage therapy for recurrent disease, 50% remained free of disease thereafter. Salvage treatment modalities varied widely and included SABR, surgery, conventional fractionated radiotherapy, concurrent chemoradiation, and chemotherapy alone. In a previous retrospective review from the Cleveland Clinic, 10 patients with local recurrence were treated with salvage SBRT using a BED of >100 Gy10 (88). With a median follow-up of 13.8 months, 4 patients (40%) were disease-free at the time of reporting, while 2 patients (20%) developed distant-only failure. In both of these studies, grade 3 toxicity was minimal. These findings emphasize the importance of close post-treatment surveillance for early detection of still salvageable and potentially curable disease recurrence. Long-term control and toxicity data, as well as further investigation into optimal dose constraints for critical structures in the setting of repeat SBRT for salvage, are needed.
Conclusions
Non-surgical definitive therapy for ES-NSCLC with radiation therapy is not yet standard but has been shown to likely be an at least comparable if not in select cases a superior modality given its non-invasive nature and more optimal toxicity profile. For patients with medically inoperable disease or who refuse surgery, definitive SBRT is an important alternative treatment option that continues to improve in terms of understanding of optimal dose-fractionation, dose constraints unique to SBRT, motion management, and plan optimization.
Given that approximately two-thirds of ES-NSCLC disease recurrences present nodally or distantly, the question of combining systemic therapy with SBRT has been a topic of recent discussion (83). In particular, the use of immunotherapy with SBRT is a compelling area of investigation, as these agents are generally well-tolerated in comparison with traditional chemotherapy drugs, and they may induce the thus-far largely hypothetical immune-mediated abscopal effect in conjunction with the use of large fraction sizes delivered in SBRT treatment (89).
Proton therapy is burgeoning in the field of radiation oncology as a modality with the potential to benefit multiple disease sites given its ability to achieve often superior normal tissue sparing due to the Bragg peak of the entering proton beam. This may not only reduce normal tissue toxicities but can also allow for safe dose escalation beyond what has been possible to date with photon SBRT (90,91). Carbon ion therapy is also under investigation as a radiation modality that may be able to further improve the therapeutic index of SBRT delivery (91). Additional prospective study is needed to further elucidate the potential role of particle therapy in the treatment of ES-NSCLC.
More mature data on the use of SBRT for ES-NSCLC are emerging, and early control and toxicity outcomes appear to be supported by longer-term follow-up. With continued analysis of these studies and emergence of new information from ongoing clinical trials, the role of SBRT in the management of ES-NSCLC will continue to become clearer, enabling more widespread acceptance of this modality in the multidisciplinary thoracic oncology setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- American Cancer Society. Global Cancer Facts & Figures 3rd Edition. Atlanta: American Cancer Society, 2015.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Cancer of the Lung and Bronchus (Invasive). In: Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute, 2017.
- Amin MB, Edge S, Greene F, et al. AJCC Staging Manual. 8th Ed. New York, NY: Springer International Publishing, 2017.
- Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag, 2010.
- Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual 6th Ed. Chicago, IL: Springer, 2002.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 3.2018). Available online: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf, accessed February 22, 2018.
- Detterbeck FC, Mazzone PG, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S.
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Smith RA, Brooks D, Cokkinides V, et al. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin 2013;63:88-105. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society, 2018.
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [Crossref] [PubMed]
- Inoue T, Shimizu S, Onimaru R, et al. Clinical outcomes of stereotactic body radiotherapy for small lung lesions clinically diagnosed as primary lung cancer on radiologic examination. Int J Radiat Oncol Biol Phys 2009;75:683-7. [Crossref] [PubMed]
- Takeda A, Kunieda E, Sanuki N, et al. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: comparison with non-small-cell lung cancer. Lung Cancer 2012;77:77-82. [Crossref] [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative Predictive Value of Positron Emission Tomography and Computed Tomography for Stage T1-2N0 Non-Small-Cell Lung Cancer: A Meta-Analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014;11:CD009519. [PubMed]
- Birim O, Kappetein A, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. [Crossref] [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Schonewolf CA, Verma V, Post CM, et al. Outcomes of invasive mediastinal nodal staging versus positron emission tomography staging alone for early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Lung Cancer 2018;117:53-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Stiles BM, Servais EL, Lee PC, et al. Point: Clinical stage IA non-small cell lung cancer determined by computed tomography and positron emission tomography is frequently not pathologic IA non-small cell lung cancer: the problem of understaging. J Thorac Cardiovasc Surg 2009;137:13-9. [Crossref] [PubMed]
- Cancer Stat Facts: Lung and bronchus cancer, 2010-2014. National Cancer Institute, Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/lungb.html, accessed on February 22, 2018.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- NRG Oncology. RTOG 0813: Seamless phase I/II study of stereotactic lung radiotherapy for early stage, centrally located, non-small cell lung cancer in medically inoperable patients. Available online: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?action=openFile&FileID=9067
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: An analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Guckenberger M, Klement RJ, Kestin LL, et al. Lack of a dose-effect relationship for pulmonary function changes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1074-81. [Crossref] [PubMed]
- Yamashita H, Onishi H, Shioyama Y, et al. Stereotactic body radiation therapy for patient with pulmonary interstitial change. Int Radiat Oncol Biol Phys 2015;90:S651-2. [Crossref]
- Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015;10:116-25. [Crossref] [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in the treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1-11. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I non-small cell lung carcinoma clinical outcome in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Boyer MJ, Williams CD, Harpole DH, et al. Improved survival of stage I non-small cell lung cancer: a VA central cancer registry analysis. J Thorac Oncol 2017;12:1814-23. [Crossref] [PubMed]
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: a National Cancer Data Base analysis. Cancer 2015;121:4222-30. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Hurkmans CW, Cuijpers JP, Lagerwaard FJ, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomized phase III ROSEL study. Radiat Oncol 2009;4:1. [Crossref] [PubMed]
- Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:688-96. [Crossref] [PubMed]
- Verma V, Shostrom VK, Zhen W, et al. Influence of Fractionation Scheme and Tumor Location on Toxicities After Stereotactic Body Radiation Therapy for Large (≥5 cm) Non-Small Cell Lung Cancer: A Multi-institutional Analysis. Int J Radiat Oncol Biol Phys 2017;97:778-85. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gasper LE, et al. Efficacy and toxicity analysis of NRG oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;96:S8. [Crossref]
- Chang JY, Bezjak A, Mornex F. Stereotactic Ablative Radiotherapy for Centrally Located Early Stage Non-Small-Cell Lung Cancer: What We Have Learned. J Thorac Oncol 2015;10:577-85. [Crossref] [PubMed]
- Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Oshiro Y, Aruga T, Tsuboi K, et al. Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlenther Onkol 2010;186:274-9. [Crossref] [PubMed]
- Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009;91:301-6. [Crossref] [PubMed]
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [Crossref] [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [Crossref] [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys 2014;88:1120-8. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Wu AJ, Williams E, Modh A, et al. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiother Oncol 2014;112:267-71. [Crossref] [PubMed]
- Stephans KL, Djemil T, Diaconu C, et al. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: risk factors for late toxicity. Int J Radiat Oncol Biol Phys 2014;90:197-202. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol 2016;6:e27-33. [Crossref] [PubMed]
- Videtic GM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys 2015;93:757-64. [Crossref] [PubMed]
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:796-801. [Crossref] [PubMed]
- Mutter RW, Liu F, Abreu A, et al. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys 2012;82:1783-90. [Crossref] [PubMed]
- Din SU, Williams EL, Jackson A, et al. Impact of Fractionation and Dose in a Multivariate Model for Radiation-Induced Chest Wall Pain. Int J Radiat Oncol Biol Phys 2015;93:418-24. [Crossref] [PubMed]
- Stephans KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol Phys 2012;82:974-80. [Crossref] [PubMed]
- Bongers EM, Haasbeek CJ, Lagerwaard FJ, et al. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J Thorac Oncol 2011;6:2052-7. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either sterotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes After Stereotactic Lung Radiotherapy or Wedge Resection for Stage I Non-Small-Cell Lung Cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2014;90:S30. [Crossref]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non-small cell lung cancer. JAMA Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031-9. [Crossref] [PubMed]
- Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 2015;10:960-4. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Matsuo Y, Shibuya K, Nagata Y, et al. Preliminary report of late recurrences, at 5 years or more, after stereotactic body radiation therapy for non-small cell lung cancer. J Thorac Oncol 2012;7:453-6. [Crossref] [PubMed]
- Henderson MA, Hoopes DJ, Fletcher JW, et al. A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:789-95. [Crossref] [PubMed]
- Zhang X, Liu H, Balter P, et al. Postiron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:1558-65. [Crossref] [PubMed]
- Hoopes DJ, Tann M, Fletcher JW, et al. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229-34. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumous: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Nguyen TK, Senan S, Bradley JD, et al. Optimal imaging surveillance after stereotactic ablative radiation therapy for early-stage non-small cell lung cancer: Findings of an International Delphi Consensus Study. Pract Radiat Oncol 2018;8:e71-e78. [Crossref] [PubMed]
- Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013;109:51-7. [Crossref] [PubMed]
- Senthi S, Lagerwaard FG, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Spratt DE, We AJ, Adeseye V, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: Implications for surveillance. Clin Lung Cancer 2016;17:177-183.e2. [Crossref] [PubMed]
- Huang K, Palma DA. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015;10:412-9. [Crossref] [PubMed]
- Verma V, Choi JI, Sawant A, et al. Use of PET and other functional imaging to guide target delineation in radiation oncology. Semin Radiat Oncol 2018;28:171-7. [Crossref] [PubMed]
- Videtic GM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Simone CB 2nd, Burri SH, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl Lung Cancer Res 2015;4:545-52. [PubMed]
- Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1015-22. [Crossref] [PubMed]
- Wink KCJ, Roelofs E, Simone CB 2nd, et al. Photons, protons or carbon ions for stage I non-small cell lung cancer - Results of the multicentric ROCOCO in silico study. Radiother Oncol 2018;128:139-46. [Crossref] [PubMed]


