Editor’s Note:
In the era of personalized medicine, a critical appraisal new developments and controversies are essential in order to derived tailored approaches. In addition to its educative aspect, we expect these discussions to help younger researchers to refine their own research strategies.
PROS: should immunotherapy be incorporated in the treatment of oncogene-driven lung cancer?
Immune checkpoint inhibitors (ICPIs) targeting the programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) axis have dramatically expanded the therapeutic armamentarium for non-small cell lung cancer (NSCLC) and are now standard treatments for patients with advanced disease (1). However, most patients with NSCLC do not respond to ICPIs, an observation motivating ongoing research efforts to identify reliable predictive biomarkers. So far, such efforts have largely centred on PD-L1 expression, although several other biomarkers are currently under investigation. A high tumour mutational burden (TMB), as defined by at least 10 mutations per megabase, has been recently identified as a possible predictive biomarker for progression-free survival (PFS) with an ICPIs combination (nivolumab plus ipilimumab) as compared to chemotherapy in the first-line treatment of patients with NSCLC (2). This finding theoretically further challenges the role of ICPIs in patients with targetable oncogenic drivers, such as epidermal growth factor receptor mutations (EGFRmut) and anaplastic lymphoma kinase rearrangements (ALK+), that is rather considered an “oligoclonal disease” (3-5). Within this group of patients, ICPIs have been understudied as they were excluded or minimally represented within randomised trials with ICPIs.
Here we examine some preclinical, translational and clinical data suggesting that ICPIs should be reasonably not excluded a priori in these patients.
Pre-clinical data showed that the EGFR oncogenic signaling may promote PD-L1 expression and treatment with PD1 inhibitors could enhance tumour responses in EGFR-mutated models (6,7). Indeed, EGFR-mutated tumors are often characterized by a high PD-L1 expression (8). However, other translational studies demonstrated that EGFR-mutated NSCLC is characterized by a low TMB (9) and a low rate of associated tumour infiltrating lymphocytes (TILs) (10), that could reduce the immune-dependence of these tumors. However, some changes could occur along the course of the disease and should be considered. PD-L1 overexpression and high rate of TILs were simultaneously detected in the tumour microenvironment of only 1/57 tyrosine kinase inhibitor (TKI)-naïve but in 5/57 TKI-resistant EGFR-mutated NSCLC patients (10). Accordingly, in a survival analysis of 25 EGFR+ NSCLC patients receiving nivolumab after EGFR-TKI, stratified according to the T790M-status, T790M-negative patients showed major benefit from nivolumab as compared to T790M positive patients (11). Furthermore, treatment with TKIs may have a proinflammatory effect. In 18 patients with EGFRmut NSCLC, paired biopsies before and after treatment with gefitinib showed increased PD-L1 expression and CD8+ T cells and association with MET positivity after treatment with this EGFR-TKI, suggesting also that rebiopsy should be considered when using the PD-L1 expression as a biomarker (12).
Similarly, preclinical evidence suggests that ALK may upregulate PD-L1 by activating PI3K-AKT and MEK-ERK signalling pathways in NSCLC (7). However, based on a systematic review and meta-analysis of 15 studies conducted on patients with tumor PD-L1 expression ≥5% (predominantly Asian), the PD-L1 positivity was more frequently found in ALK-negative patients (OR 388.6; 95% CI, 222.5–678.7; P<0.001), with only 8% of ALK-positive patients showing a PD-L1 expression (13). In the same paper, a similar frequency of ALK (4.4%) and ROS1 translocations (1.7%) to that usually reported in the general population was found in the subgroup of patients with tumor PD-L1 expression ≥5% (13).
Clinically, an initial retrospective series of 58 patients reported that EGFRmut or ALK rearrangements were associated with low overall response rates (ORR) to PD-1/PD-L1 ICPIs (10). A retrospective pooled analysis of four randomized studies comparing ICPIs vs. docetaxel in pre-treated patients with advanced NSCLC and a meta-analysis demonstrated no differences in overall survival (OS) between ICPIs and chemotherapy in EGFR-mutant NSCLC patients (14-18), suggesting that patients with EGFRmut NSCLC may not be the good candidate to ICPIs. In these trials (14,16,17), the ALK+ populations were not specifically examined and no related data on OS are available. However, several papers are now coming out with meaningful information regarding the possible activity of ICPIs even in NSCLC patients with driver mutations.
The first one is the Atlantic study (19), a phase 2 trial evaluating the activity of the PD-L1 inhibitor durvalumab in advanced NSCLC after at least two lines of systemic therapy. In this study, patients were enrolled into three cohorts based on their EGFR/ALK status and tumour cell expression of PD-L1: cohort 1, EGFR+/ALK+ NSCLC with at least 25% of tumour cells expressing PD-L1; cohort 2, EGFR−/ALK− NSCLC with at least 25% of tumour cells expressing PD-L1; and cohort 3, EGFR-/ALK− NSCLC with at least 90% of tumour cells expressing PD-L1. Initially, patients were enrolled regardless of PD-L1 status, but a subsequent protocol amendment restricted study entry to patients with PD-L1 positive tumors (i.e., at least 25% of cells positive for PD-L1 at any staining intensity); therefore, cohorts 1 and 2 also included some patients with less than 25% of tumour cells expressing PD-L1. Objective responses, according to an independent central review in evaluable patients, were rather similar between the cohort 1 and 2, being observed in nine of 74 patients (12.2%) and 24 of 146 (16.4%), respectively, and higher in cohort 3, in 21 of 68 patients (30.9%). The activity of durvalumab in patients with EGFR+/ALK+ NSCLC with low PD-L1 expression was minimal, with only one (4%) of these 28 patients in cohort 1 achieving an objective response. Noteworthy, 74% of patients (315 out of 425) with EGFR+/ALK+ NSCLC screened in the Atlantic study had low PD-L1 expression (<25% of tumour cells expressing PD-L1), whereas 14.6% (62 out of 425) had at least 90% of tumour cells expressing PD-L1 and represented the 42% of patients (47 out of 111) with EGFR+/ALK+ NSCLC actually treated in cohort 1. Out of 10 responders with EGFR+ NSCLC, 6 were current or former smokers, and 8 had a high PD-L1 expression (90–100% of tumour cells expressing PD-L1). Indeed, irrespective of EGFR or ALK status, median OS was higher in patients with at least 25% of tumour cells expressing PD-L1 (approximately 11–13 months) than those patients with less than 25% of tumour cells expressing PD-L1 (approximately 9–10 months). Taken together these data suggest that in pre-treated patients with EGFR+/ALK+ NSCLC PD-L1 may be overexpressed in approximately a quarter of patients and its overexpression may predict the activity of ICPIs. Although modest, this activity seems not inferior to that expected in patients with EGFR−/ALK− NSCLC, especially in those patients with very high PD-L1 overexpression (90–100% of tumour cells expressing PD-L1), confirming that immune-based approaches might still be viable options for progressive oncogene-driven NSCLC selected on predictive biomarkers.
The second evidence is provided by the results of the phase II BIRCH study that have recently demonstrated a relevant activity in terms of overall response rate of atezolizumab monotherapy as first-line or subsequent therapy in PD-L1+ (with ≥5% expressing tumor or immune cells) NSCLC patients, regardless of tumor EGFR or KRAS mutation status (20). This study treated 45 patients EGFRmut, 137 with KRAS mutations and 9 patients with ALK alterations. Patients with EGFR+/ALK+ NSCLC must have had disease progression or intolerance to an EGFR or ALK TKIs.
Third evidence comes from the IMpower150 trial (21) reporting a significantly improved PFS in the general population of 800 patients (of 8.3 vs. 6.8 months, HR 0.61) and in the subgroup of 108 evaluable patients with TKI pre-treated EGFR+ (80 patients)/ALK+ (34 patients) NSCLC (of 9.7 vs. 6.1 months, HR 0.59) with the addition of atezolizumab to chemotherapy with carboplatin, paclitaxel and bevacizumab vs. chemotherapy plus bevacizumab.
However the Atlantic study showed the possible difference in the activity of ICPIs between EGFRmut and ALK+ NSCLC. Although in the study (19) patients with EGFRmut and ALK+ NSCLC were grouped together in the same cohort 1, results suggest that differences may exist and these molecular subsets might have distinct immunobiology. Patients tested and screened for the study with ALK+ NSCLC showed higher frequency of PD-L1 overexpression than those with EGFRmut NSCLC, with 48.5% (16 out of 33) vs. 24.3% (87 out of 358) of them having at least 25% of tumour cells expressing PD-L1 and 30.3% (10 out of 33) vs. 13.4% (48 out of 358) having at least 90% of tumour cells expressing PD-L1, respectively. The activity of durvalumab was concentrated solely in the EGFRmut group. No objective responses were recorded in patients with ALK+ NSCLC, although the number of patients with ALK+ NSCLC treated was really small (15 patients). This finding highlights even more that PD-L1 expression on its own is an imperfect biomarker and needs to be complemented by other factors, such as TMB and TILs for instance. On the other hand, in another NSCLC molecular subtype, that is not still amenable to targeted therapies, the KRAS mutated, a meta-analysis of three randomized trials (with the data of OS stratified by KRAS mutation status) showed that ICPIs as salvage therapy improved OS compared to docetaxel in advanced NSCLC patients with KRAS mutation, but not in those with KRAS wild-type (22). Furthermore, in the BIRCH phase II study, ORR with atezolizumab was even higher in the 137 patients with KRAS mutations (28% of all enrolled patients) as compared to those with wild-type tumors, being 27% vs. 16%, 32% vs. 16% and 19% vs. 18% in first-line, second-line and ≥ third-line, respectively (20). Since high TMB is often associated with KRAS + NSCLC (23), these results may suggest again that the presence of the oncogenic driver may not be alone the determinant of the response to ICPIs.
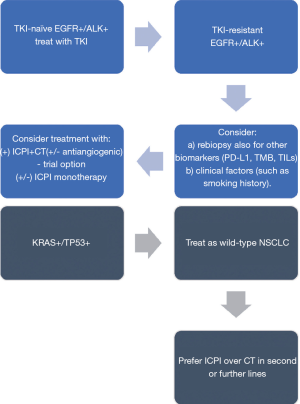
Based on available data here presented, we would suggest the following conclusions summarized in Figure 1. Firstly, biomarkers predictive of “inflamed” or “immune-sensitive” tumors (such as, PD-L1 overexpression, TMB and TILs), even still imperfect, along with clinical factors (such as smoking history), should have more relevance than the oncogenic driver for the decision-making of further treatments following TKIs in oncogene-addicted NSCLC. Second, patients with oncogene-addicted NSCLC should not be excluded a priori from the participation to clinical trials with ICPIs, neither in clinical practice, when a TKI resistance is developed. In particular, ICPI in combination with chemotherapy (with the possible addition of an antiangiogenic agent) seems the most promising approach deserving further investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Melosky B, Chu Q, Juergens R, et al. Pointed Progress in Second-Line Advanced Non-Small-Cell Lung Cancer: The Rapidly Evolving Field of Checkpoint Inhibition. J Clin Oncol 2016;34:1676-88. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Pilotto S, Rossi A, Vavala T, et al. Outcomes of First-Generation EGFR-TKIs Against Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Post Hoc Analysis of the BE-POSITIVE Study. Clin Lung Cancer 2018;19:93-104. [Crossref] [PubMed]
- Addeo A, Tabbo F, Robinson T, et al. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit Rev Oncol Hematol 2018;122:150-6. [Crossref] [PubMed]
- Banna GL, Tiseo M. How to develop novel treatments for EGFR-mutant lung cancer. Future Oncol 2015;11:2375-8. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- D'Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34:9017. [Crossref]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 2017;28:1532-9. [Crossref] [PubMed]
- Han JJ, Kim DW, Koh J, et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:263-70.e2. [Crossref] [PubMed]
- Petrelli F, Maltese M, Tomasello G, et al. Clinical and Molecular Predictors of PD-L1 Expression in Non–Small-Cell Lung Cancer: Systematic Review and Meta-analysis. Clinical Lung Cancer 2018;19:315-22. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Reck M, Socinski MA, Cappuzzo F, et al. 134PD Primary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel +/− bevacizumab, with or without atezolizumab in 1L non-squamous metastatic NSCLC (IMpower150). J Thorac Oncol 2018;13:S77-8. [Crossref]
- Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A meta-analysis and review. Oncotarget 2017;8:48248-52. [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]


