Combined modality treatment in mesothelioma: a systemic literature review with treatment recommendations
Introduction
It was first suggested in 1980 that selected patients with malignant pleural mesothelioma (MPM) are to be treated with a combination of different therapeutic modalities, usually surgery, chemotherapy (CT) and radiotherapy (RT), in order to achieve locoregional control and to improve overall outcome (1). Other types of neoplasms that are treated in this manner include lung (2), esophageal (3) and colorectal cancer (4).
As we seem to reach consensus on the necessity of such a multimodality approach or at least on the need for more randomized trials ascertaining its role in the treatment of MPM, there is ongoing debate and research on its individual components and how they should be sequenced. Historically, surgery for MPM consisted of extrapleural pneumonectomy (EPP), which is an en bloc resection of both the parietal and visceral pleurae, the ipsilateral lung as well as pericardium and diaphragm. In the last five years there has been an evolution towards pleurectomy/decortication (P/D), which is a less invasive procedure whereby the parietal and visceral pleurae are removed but lung, pericardium and diaphragm are spared. When diaphragm and pericardium are also resected, this procedure is called an extended P/D (eP/D). Partial pleurectomy is performed to obtain tissue for diagnosis or to relieve symptoms in a palliative setting but is not part of a multimodality treatment for MPM (5).
In 2003 and 2005, two large scale randomized control trials showed superiority in overall survival time with cisplatin and pemetrexed/raltitrexed over monotherapy with cisplatin, as well as in time to progression and response (6,7). Since then this combination of cytotoxic agents (platinum based plus antifolate) has become the standard of care, as it is the sole treatment with proven benefit on outcome. In 2015, the French MAPS trial showed that the association of bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor A (VEGF-A), to a cisplatin-pemetrexed backbone improved overall survival in highly selected patients (8).
Concerning radical RT there is an ongoing effort to find the most effective radiation technique that will improve locoregional disease control but will not result in excess radiation pneumonitis. Because of the large surface area of the pleura, the volume with conventional RT is very high, resulting in damage to the underlying lung (in case of P/D) and/or surrounding tissue and organs. Intensity modulated radiotherapy (IMRT) aims at a better dose distribution and hence less toxicity, which is especially important when RT is administered after lung sparing surgery (9).
The 2010 European Respiratory Society (ERS)-European Society of Thoracic Surgeons (ESTS) guidelines, recommend that eligible patients (early stage, preferably epithelioid subtype, satisfactory pulmonary and cardiac status) should be included in prospective randomized control trials evaluating multimodality treatment in experienced centers (10). The 2015 European Society for Medical Oncology (ESMO) guidelines are more restrictive as they conclude that there is insufficient evidence supporting standardized implementation of adjuvant RT in the treatment of MPM, however they do advise against monotherapy with surgery and recommend surgery be part of a multimodality treatment, preferably in a trial setting (11). The 2018 British Thoracic Society (BTS) advise against EPP in any way and against eP/D outside of clinical trials. Based on the results of the MAPS trial, they suggest association of bevacizumab to a platinum-antifolate doublet to improve survival (8,12). The 2018 American Society of Clinical Oncology (ASCO) guidelines state that cytoreductive surgery should ideally be supplemented by RT or (neo)adjuvant CT seeing as surgery alone has not shown to provide adequate disease control. For patients with proven N2 status they only recommend surgery when part of a multimodality treatment, preferably in a trial setting (13). An overview of the most recent guidelines is listed in Table 1.
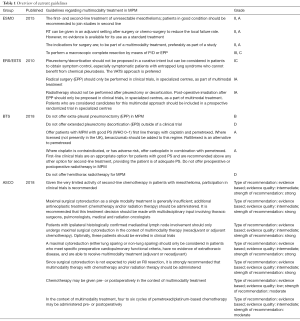
Full table
The purpose of this manuscript is to report the results of a systematic literature search performed on the subject of combined modality treatment in mesothelioma and formulate graded recommendations.
The following clinical questions were raised:
- Is multimodality treatment better than CT alone?
- What is the optimal regimen within each modality?
- What is the optimal sequence of interventions within a combined modality approach?
Methods
This systematic review is part of the revision of the 2010 ERS-ESTS guidelines on mesothelioma by a core group of experts, led by Prof Dr. A Scherpereel. A literature search was performed in November 2016 in the Ovid Medline system by a librarian. The clinical questions were translated in the “PICO” (population, intervention, comparator, outcome) model (14) (Table 2). The corresponding search criteria were translated into MeSH terms, and free-text keywords that were searched for in titles, abstracts and name of substances (Table S1). Completed search strategies included “P” and “I” criteria, combined with “O” criteria when the number of retrieved citations was too large. Results were limited to articles published from 2009 onwards. Citations were exported from Medline into reference manager databases to allow the removal of duplicates and to facilitate the selection process performed by the reviewers. They were first selected for their eligibility based on the abstract content and language. The remaining articles were evaluated further for inclusion in the current review. The final selection was performed by reading the full publication. Selection was independently done by the four authors and discrepancies were consensually resolved. This search was supplemented by screening the references of the selected articles and a manual selection of literature up to January 2018 by the experts. An evidence level and grade of recommendation was assigned using the SIGN methodology.

Full table
Results
We withheld out of 80 abstracts, 35 valid full articles, of which 9 reviews/editorials/comments, 5 guidelines, 9 manuscripts addressing pure surgical issues, 7 population studies, 4 manuscripts on recurrence pattern after combined modality and 1 case report (for grading see Table S1). Among these 35 articles, there was 1 observational study on single modality treatment, 1 phase II trial on single modality treatment (CT), 3 randomized phase III trials on single modality treatment (of which 2 on CT and 1 on immunotherapy), there were 12 reports on 2 modalities, of which 3 prospective, non-randomized series, 7 on surgery with CT (of which 2 with intracavitary CT): 1 observational, 1 phase 2 trial, 5 on peri-operative RT of which 1 prospective; 17 reports on 3 modalities among which 1 systematic review, 1 retrospective analysis, 7 phase 2 trials, 2 randomized trials; 7 on PORT; 1 on resection. These were the basis for addressing the three PICO questions.

Full table
PICO 1: is multimodality treatment better than CT alone?
Primary endpoint: survival
Two groups have analyzed the pooled mesothelioma data in the National Cancer Database (NCDB), wherein MPM patients’ records are collected. Both report that almost half of the patient population received no mesothelioma specific treatment. Saddoughi et al. found that the 3 percent of MPM patients who underwent multimodality treatment between 2004–2013 performed better than those treated with CT alone (median overall survival 19.9 vs. 11.3 months, respectively) (15). Nelson et al. confirmed the use of multimodality treatment in only 8% of seemingly ideal trimodality treatment patients (under 70 years old, stage I through III, epithelioid histology) (16).
Although some reports are in favor of a multimodal approach when it comes to the treatment of MPM, others have shown similar or even better overall survival treating patients with CT alone. The explanation for this lies in the morbidity and mortality associated with EPP.
Hillerdal et al. treated a series of patients with MPM with a combination of carboplatin, liposomal doxorubicin and gemcitabine. They found an overall median survival of 13 months. Epithelioid subtypes reached a median survival of 17 months and even 21 months in good performance status patients, comparable with the outcome of patients treated with neoadjuvant CT and EPP, suggesting that treatment with CT alone is equally effective in similarly selected MPM patients (17).
Sharkey et al. retrospectively analyzed their database in order to establish the ideal timing for CT in patients treated with EPP and to identify which MPM patients benefit most from (neo)adjuvant CT. Their results showed a median survival from time of diagnosis of 23.3 months in the adjuvant CT group and 23.9 months in the neo-adjuvant group. A scenario wherein no CT was given until disease progression, performed similarly with a median survival of 20.3 months. Those patients with non-epithelioid histology and nodal involvement performed better with true adjuvant CT than when it was delayed (overall survival 15.6 vs. 8.2 months and progression free survival 14.9 vs. 6.0 months respectively) (18).
The Mesothelioma and Radical Surgery (MARS) trial, which randomized eligible patients after 3 cycles of induction CT to either EPP or postoperative radiotherapy (PORT) versus 3 more cycles of CT without attempt at resection or RT, concluded that the likelihood of a benefit of EPP on the overall survival and quality of life endpoints was small. This futility analysis caused the premature closure of the trial and affected standard practice worldwide (19). Median survival of the EPP group (14.4 months) was low compared to 19.5 months in the no EPP group, but also lower than in other similarly designed historical EPP trials. The start date of the time-to-event analysis—after induction CT—is the most likely explanation for this comparably low outcome, as in other trials this was calculated from the time CT was started.
Additionally, overall survival reported in the CT arm of the MARS trial is significantly better than that reported in certain multimodality treatment series. Bille et al. included 25 patients in a prospective study examining the effects of trimodality treatment and reported a median survival of only 12.8 months (20).
Although intertrial comparisons are hazardous, these results do not strengthen the role of surgery in the treatment of MPM, especially when considering the time patients undergoing a multimodality treatment spend hospitalized after surgery or due to complications of treatment.
The MARS group is currently assessing the feasibility of a study comparing CT alone versus CT and eP/D. Patients will be randomized to either eP/D or no surgery after completing 3 cycles of CT (21).
The addition of RT to surgery has also been studied, with variable results. The SAKK 17/04 trial, a prospective randomized trial, assessed whether adding PORT to a combination of neo-adjuvant CT (cisplatin-pemetrexed) and EPP resulted in a better locoregional relapse-free survival (22). Although they showed a significantly longer local relapse free survival in the RT arm (9.4 vs. 7.6 months), the authors were unable to make a convincing case for the addition of PORT to CT and surgery when it comes to median survival seeing as it was similar to the no-RT arm, namely 19.3 vs. 20.8 months respectively. The addition of PORT also comes at a price, as there was a radiation related death in the SAKK PORT arm, as opposed to no treatment related mortality in the SAKK no PORT arm.
These findings were similar to those published by a Japanese group performing a feasibility study with a comparable trimodality protocol (CT-EPP-RT) in mesothelioma patients. Median survival in this patient population was 19.9 months and although they met the primary endpoints of achieving a macroscopic complete resection and maintaining an acceptable treatment related mortality, they concluded that the risk-benefit ratio was unsatisfactory. Progression free survival time was 11 months in those wherein a macroscopic complete resection was achieved (23).
It would be premature however to exclude RT from the treatment of MPM altogether based on these results alone. It is plausible that more rigorous patient selection and fine-tuning of the techniques could result in better outcomes and less toxicity. The SAKK trial included patients with extensive disease (proven N2 status) and non-epithelioid type mesotheliomas, both of which are negative prognostic features usually serving as exclusion criteria in this type of trial. The RT regimen in both trials also consisted of hemithoracic RT, which is associated with a high radiation dose and hence high toxicity. EPP was the surgical procedure of choice in both trials, which has a high mortality and is prone to complications. In this light, the results of the JMIG1101 trial, a prospective feasibility study of induction CT followed by P/D will be of particular interest.
A recent systematic review of randomized controlled trials of multimodality treatment in MPM (namely MARS and SAKK trials), concluded that based on this data there is not enough evidence to support standardized implementation of a combined modality treatment, especially given the morbidity and cost associated with these treatment protocols (24).
In conclusion, the current evidence in favor of a combined modality approach is weak and likely subject to different biases. Nevertheless, the rationale is challenging and the best survival data reported were obtained in series combining local and systemic modalities.
Other endpoints
Marulli et al. did a retrospective analysis of mesothelioma patients who received neo-adjuvant CT and found that it improved their pulmonary lung function tests and exercise capacity (25). Not only should this affect quality of life, this could also prime patients before surgery, possibly resulting in lower surgery-associated morbidity.
Maintaining a satisfactory physical condition is essential for completing these demanding treatment schedules. Exactly how challenging these therapies are, is revealed in the number of patients completing the treatment protocols, or the time it takes to reach completion versus an optimal preset timeline. In the EORTC 08031 study, a prospective feasibility study assessing multimodality treatment (CT-EPP-PORT) in the treatment of early stage MPM, only 42% of enrolled patients were able to complete the treatment within the preset time frame (26). In the aforementioned SAKK trial, only 36% of patients were able to or agreed to proceed on to RT after undergoing neo-adjuvant CT and EPP (22). So, attrition is high and compliance to treatment becomes an important endpoint. Omitting to report this attrition is common in retrospective series and is the cause for the commonly encountered immortality bias in combined mortality series.
Toxicity is systematically underreported thus minimalizing the morbidity associated with multimodality treatment schedules. Toxic or treatment-related deaths are most often the result of complications brought on by surgery or radiation. In the SMART trial (IMRT followed by EPP and CT), de Perrot et al. report that 24 out of 62 patients developed a grade 3 or higher toxicity. This number does not account 6 patients with multiple grade 3 or higher toxicities, summing up to 31 the overall number of single serious adverse events in this patient group (27). In their series, Federico et al. had to reduce the radiation dosage after two patients died due to radiation related cardiopulmonary complications (28). Even after this dose reduction, the grade 3-4 toxicity incidence remained high at 66.7% of patients. Note that this is again the percentage of patients developing one or more grade 3–4 toxicities, not the number of single serious adverse events.
On occasion, adverse events are reported per treatment modality (e.g., CT related toxicity) instead of an overall number of adverse events (22,23,29). This too can lead to an underestimation of the morbidity inflicted by the entire treatment regimen.
Table 3 shows the toxic deaths reported in the prospective multimodality treatment trials. As these are mostly complications of local treatment, improvements should be made in these areas in order to reduce mortality.

Full table
Unfortunately, neither resection nor RT currently results in adequate locoregional disease control. Cao et al. pooled the results of all major trimodality treatment trials and found disease relapse to occur most often locally, with an incidence ranging between 4–41% (32).
In conclusion, a combined modality approach leads to accumulated toxicity and mortality which is the main reason for the failure of CMT to show an unequivocal benefit in outcome. Any approach reducing the latter might well result in an improved survival and adoption of CMT.
PICO 2: what is the optimal regimen within each modality?
What is the optimal PORT-technique?
RT of the pleura is understandably challenging as there is a large surface area to irradiate, the shape is complex and there are vital organs and large vascular structures close to the pleura that need to be shielded from radiation as much as possible (33). The evolution towards lung sparing surgical techniques introduces additional difficulty as measures must be taken to avoid radiation pneumonitis or other complications in the spared ipsilateral as well as in the contralateral lung. The most frequently used radiation technique in multimodality treatment protocols for MPM are conventional 3-dimensional conformal RT and intensity modulated RT (IMRT). Conventional 3D hemithoracic RT has shown to be excessively toxic by Gupta et al. and Stahel et al. among others, both in the context of lung sparing surgery and after EPP (22,34). IMRT allows for a tailored approach wherein large doses of radiation are administered to the affected pleura and the underlying lung and surrounding tissue are spared (35). Helical tomotherapy or dynamic arc RT combines the precision of the IMRT technology with a megavoltage CT-scan. This allows for daily image-guided adjustments, which creates a more precise application of radiation. The fact that the radiation beam moves around the patient while the table moves through the arc enables irradiation of larger areas in a shorter time period (36). Sylvestre et al. applied this technique for MPM in 24 MPM patients after EPP and reported a median disease free survival of 24 months. Two patients died of radiation pneumonitis (36). Krayenbuehl et al. compared IMRT and 3D conformal RT in 39 MPM patients after EPP. They found a non-significantly longer median time to relapse in the IMRT group (16.2±3.1 versus 10.9±5.4 months with 3DRT). This did not however result in a longer overall survival (22.3±15.3 months for IMRT and 21.2±9.2 months for 3DCRT), probably because of a higher rate of distant relapse (33). Rimner et al. evaluated IMRT after CT and P/D and reported a median progression free survival of 12.4 months and a very promising median overall survival of 23.7 months. There were no treatment related deaths. There were 8 cases (30% of patients) of grade 2–3 pneumonitis which responded well to steroid treatment (35). In conclusion, IMRT techniques are hence likely to be preferable to 3DRT with regards to toxicity.
What is the optimal CT regimen?
The standard CT regimen in multimodality treatment for MPM is a cisplatin-pemetrexed doublet. Pasello et al. performed a retrospective analysis of 51 patients who received neoadjuvant CT (pemetrexed plus cisplatin or carboplatin) as part of a multimodality treatment approach for MPM. Although they report a similar median progression free survival outcome in both groups (14.5 months in the carboplatin group versus 13.1 months in the cisplatin group), overall survival was significantly longer in the carboplatin group (25.5 versus 15.2 months in the cisplatin group). The authors attribute this to the patients’ characteristics being more favorable in the carboplatin group. When they compared outcomes for only epithelioid-type mesothelioma in both groups, the difference was not statistically significant (26.9 months in the carboplatin group versus 18.9 months in the cisplatin group, P=0.054). As expected, cisplatin was tolerated more poorly and treatment with cisplatin resulted in higher numbers of anemia, nausea, vomiting and asthenia as opposed to carboplatin-based therapy (37). A Turkish series, in which patients were treated with a pemetrexed-carboplatin doublet similarly showed a better median survival than with a pemetrexed-cisplatin doublet (38).
In conclusion, these reports suggest that carboplatin containing regimens could be equally effective and less toxic and thus preferable in a combined modality approach already hampered by other toxicities.
PICO 3: what is the optimal sequence of combined modality?
Neo-adjuvant vs. adjuvant CT
Advocates for neoadjuvant CT claim a better tolerance, compliance and resectability with this approach (22,26,28,29). In their retrospective series, Sharkey et al. however, did not find a difference in overall survival between the adjuvant CT group and the neo-adjuvant CT group (18).
Cao et al. reported a median overall survival of 23.1 months in the adjuvant CT group versus 27.8 in the neo-adjuvant CT group (32). Important to note here is that overall survival was estimated from different starting points in the different trials, which undoubtedly affects the entire analysis. Also, the trials assessing adjuvant CT are older than the neo-adjuvant series and -with one exception- all of retrospective nature. The adjuvant CT regimens differed between the trials so comparing them as a group to the more homogenous neo-adjuvant CT trials is presumptuous.
In conclusion, both approaches are defendable. The European Organization for the Research and Treatment of Cancer (EORTC) is currently enrolling (cT1–3 N0–2 M0) patients for its 1205 trial wherein they are randomized to P/D, preceded or followed by 4 cycles of platinum-pemetrexed.
Pre-op vs. post-op RT
Cho et al. sought to reduce disease relapse—thought to be caused by tumor soiling during EPP—by implementing a short course of IMRT prior to EPP. Ideally, both a direct tumoricidal effect and a distal immunomodulating or abscopal effect are generated to prevent tumor growth in distant sites. Neoadjuvant RT was well tolerated, without severe toxicity or mortality. After five years, only one out of 9 patients with epithelioid histology and no N2 involvement had relapsed (39). De Perrot et al. confirmed these results in the prospective SMART trial with an encouraging median survival of 36 months.
Discussion
We recognize several flaws in the reporting of combined modality treatment series. As malignant mesothelioma is a relatively rare disease, the volume of patients being treated in any given center is low. A small number of specialized institutions tend to dominate the publications and as a result, the published outcomes do not mirror the real-life outcomes. Recording data in (inter)national registries could help in overcoming this selection bias.
Treatment protocols also tend to be amended and adapted depending on findings during therapy, e.g., PORT is sometimes added based on operative findings. This lack of standardization makes retrospective comparison of different series hazardous.
Aside from this heterogeneity in treatment protocols, there is also the issue of bias in the definition of resectable disease. The availability of experienced surgeons or radiotherapists plays an important role in this interpretation. Patient characteristics such as N2 disease and histological subtypes are also not routinely documented and considering how significant these prognostic factors are, omitting them is casting a bias on the results.
Time-to-event outcomes such as median overall survival and progression free survival, are estimated from different starting points in different trials, making comparison between individual series and trials difficult and confusing.
Retrospective series suffer from immortal time or guarantee time bias. This arises when the analysis includes the time period before or in between treatment(s) as part of follow-up, as is often the case in multimodality treatment trials (40,41). Nelson et al. attempted to overcome this by implementing propensity matched analysis when studying the National Cancer Database (42). Vogl pointed out that this analysis is still biased in favor of the multimodality treatment group. A tell-tale sign of this statistical wizardry is the fact that the survival curve of the multimodality treatment group starts out flat. Only the patients that complete the entire treatment protocol are included in the analysis. As they have to be alive to undergo the different treatment modalities any patient who drops out of the trial ahead of completion is not taken into account when analyzing the treatment group. As a result an overestimation will occur favoring the treatment group. Unfortunately, in any disease with a prognosis as dismal as that of malignant mesothelioma, patients will die in between treatment modalities or even before any treatment is given, as such a plateau in the survival graph is inaccurate and misleading (41).
Conclusions & recommendations
Disappointingly, despite previous evidence-based guidelines and recommendations, most patients are still being treated outside of clinical trials. In this review, we found that between 2009 and 2016, only 104 patients were treated in randomized controlled trials. As such, important questions remain unanswered. The role of surgery is still up for debate as even pleurectomy/decortication scores low on the benefit to cost ratio. Novel techniques in RT and CT are promising and should be investigated further, both as monotherapy and as part of combined modality treatment. The optimal sequencing of different modalities has yet to be identified.
Answers to a number of secondary questions have been indirectly obtained from case control series and these issues should therefore not be prioritized for investigation in upcoming trials. Examples are the equivalence of carboplatin to cisplatin when it comes to efficacy, the benefit of adding CT to surgery in improving outcome and the role of advanced precision RT techniques for avoiding toxicity.
We recommend that further reports on multimodality therapy in MPM should unequivocally include an intention-to treat population analysis, report time-to-outcome measurements from day 1 of the first treatment and confer a CONSORT diagram of patient disposition over the different steps of the multimodality protocol.
Furthermore, decisions to step up or down in a combined modality approach should be agreed upon before treatment starts and not ad hoc based on intraoperative or durante treatment findings, obscuring the true effect of each modality.
We emphasize the role of well conducted randomized phase 2 trials including a control group in order to compensate for patient selection bias and to avoid embarking on large phase 3 trials based on immature results in single arm phase 2 trials.
Lastly, it is imperative that good performance patients be referred to high volume expert centers in order to be considered and proposed for the clinical trials necessary to take forward the issue of multimodality treatment on the outcome in MPM.
Recommendations
- There is level 2 evidence that a multimodality treatment consisting of at least macroscopic resection and CT, is superior to either single modality in selected patients with regard to survival, but at the cost of increased treatment related morbidity and mortality. Selected patients should be adequately informed and referred to expert centers in order to be included in either clinical trials or large institutional series (grade of recommendation: D).
- There is level 2+ evidence that for PORT, IMRT techniques should be preferred over conventional 3D RT (grade of recommendation: C).
- There is level 3 evidence that a carboplatin-based platinum-pemetrexed doublet is non-inferior to a cisplatin based one as a (neo-)adjuvant CT regimen with less toxicity (grade of recommendation: D).
- There is level 2 evidence for neo-adjuvant CT to be preferred over adjuvant CT in a multimodality approach (grade of recommendation: D).
- No recommendation can be made regarding the optimal sequencing of RT in a combined modality treatment protocol.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Antman KH, Blum RH, Greenberger JS, et al. Multimodality therapy for malignant mesothelioma based on a study of natural history. Am J Med 1980;68:356-62. [Crossref] [PubMed]
- Tabchi S, Kassouf E, Rassy EE, et al. Management of stage III non-small cell lung cancer. Semin Oncol 2017;44:163-77. [Crossref] [PubMed]
- Li S, Liu H, Diao C, et al. Prognosis of surgery combined with different adjuvant therapies in esophageal cancer treatment: a network meta-analysis. Oncotarget 2017;8:36339-53. [PubMed]
- Allaix ME, Fichera A. Modern rectal cancer multidisciplinary treatment: the role of radiation and surgery. Ann Surg Oncol 2013;20:2921-8. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v31-9. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-i30. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Sackett DL SS, Richardson WS, et al. Evidence-Based Medicine. How to Practice and Teach EBM. Asking Answerable Clinical Questions. 2nd ed. Edinburgh: Churchill Livingstone, 2000.
- Saddoughi SA, Abdelsattar ZM, Blackmon SH. National Trends in the Epidemiology of Malignant Pleural Mesothelioma: A National Cancer Data Base Study. Ann Thorac Surg 2018;105:432-7. [Crossref] [PubMed]
- Nelson DB, Rice DC, Niu J, et al. Predictors of trimodality therapy and trends in therapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2018;53:960-6. [Crossref] [PubMed]
- Hillerdal G, Sorensen JB, Sundstrom S, et al. Treatment of malignant pleural mesothelioma with carboplatin, liposomized doxorubicin, and gemcitabine: a phase II study. J Thorac Oncol 2008;3:1325-31. [Crossref] [PubMed]
- Sharkey AJ, O'Byrne KJ, Nakas A, et al. How does the timing of chemotherapy affect outcome following radical surgery for malignant pleural mesothelioma? Lung Cancer 2016;100:5-13. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Bille A, Belcher E, Raubenheimer H, et al. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: experience of Guy's and St Thomas' hospitals. Gen Thorac Cardiovasc Surg 2012;60:289-96. [Crossref] [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Hasegawa S, Okada M, Tanaka F, et al. Trimodality strategy for treating malignant pleural mesothelioma: results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int J Clin Oncol 2016;21:523-30. [Crossref] [PubMed]
- Abdel-Rahman O, Elsayed Z, Mohamed H, et al. Radical multimodality therapy for malignant pleural mesothelioma. Cochrane Database Syst Rev 2018;1. [PubMed]
- Marulli G, Rea F, Nicotra S, et al. Effect of induction chemotherapy on lung function and exercise capacity in patients affected by malignant pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:1464-9. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Minatel E, Trovo M, Bearz A, et al. Radical Radiation Therapy After Lung-Sparing Surgery for Malignant Pleural Mesothelioma: Survival, Pattern of Failure, and Prognostic Factors. Int J Radiat Oncol Biol Phys 2015;93:606-13. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37. [PubMed]
- Krayenbuehl J, Dimmerling P, Ciernik IF, et al. Clinical outcome of postoperative highly conformal versus 3D conformal radiotherapy in patients with malignant pleural mesothelioma. Radiat Oncol 2014;9:32. [Crossref] [PubMed]
- Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2005;63:1045-52. [Crossref] [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Sylvestre A, Mahe MA, Lisbona A, et al. Mesothelioma at era of helical tomotherapy: results of two institutions in combining chemotherapy, surgery and radiotherapy. Lung Cancer 2011;74:486-91. [Crossref] [PubMed]
- Pasello G, Marulli G, Polo V, et al. Pemetrexed plus carboplatin or cisplatin as neoadjuvant treatment of operable malignant pleural mesothelioma (MPM). Anticancer Res 2012;32:5393-9. [PubMed]
- Emri S, Hurmuz P, Kadilar C, et al. Pemetrexed-carboplatin doublets showed better median survival than pemetrexed-cisplatin in the treatment of Turkish malignant pleural mesothelioma patients. J Thorac Oncol 2011;6:S1371.
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [Crossref] [PubMed]
- Levesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. [Crossref] [PubMed]
- Vogl SE. Guarantee-Time Bias and Benefits of Surgery for Pleural Mesothelioma. J Clin Oncol 2018;36:624-5. [Crossref] [PubMed]
- Nelson DB, Rice DC, Niu J, et al. Long-Term Survival Outcomes of Cancer-Directed Surgery for Malignant Pleural Mesothelioma: Propensity Score Matching Analysis. J Clin Oncol 2017;35:3354-62. [Crossref] [PubMed]
- Baldini EH, Richards WG, Gill RR, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015;149:1374-81. [Crossref] [PubMed]
- Barbieri PG, Marinaccio A, Ferrante P, et al. Effects of combined therapies on the survival of pleural mesothelioma patients treated in Brescia, 1982-2006. Tumori 2012;98:215-9. [Crossref] [PubMed]
- Bece A, Tin MM, Martin D, et al. Hemithoracic radiation therapy after extrapleural pneumonectomy for malignant pleural mesothelioma: Toxicity and outcomes at an Australian institution. J Med Imaging Radiat Oncol 2015;59:355-62. [Crossref] [PubMed]
- Bolukbas S, Eberlein M, Schirren J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol 2012;7:900-5. [Crossref] [PubMed]
- Bolukbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer 2011;71:75-81. [Crossref] [PubMed]
- Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5; discussion 876. [Crossref] [PubMed]
- Chance WW, Rice DC, Allen PK, et al. Hemithoracic intensity modulated radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma: toxicity, patterns of failure, and a matched survival analysis. Int J Radiat Oncol Biol Phys 2015;91:149-56. [Crossref] [PubMed]
- Ebara T, Kawamura H, Kaminuma T, et al. Hemithoracic intensity-modulated radiotherapy using helical tomotherapy for patients after extrapleural pneumonectomy for malignant pleural mesothelioma. J Radiat Res 2012;53:288-94. [Crossref] [PubMed]
- Fahrner R, Ochsenbein A, Schmid RA, et al. Long term survival after trimodal therapy in malignant pleural mesothelioma. Swiss Med Wkly 2012;142. [PubMed]
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65; discussion 1665-7.
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6. [Crossref] [PubMed]
- Kimura T, Doi Y, Nakashima T, et al. Clinical experience of volumetric modulated arc therapy for malignant pleural mesothelioma after extrapleural pneumonectomy. J Radiat Res 2015;56:315-24. [Crossref] [PubMed]
- Klikovits T, Hoda MA, Dong Y, et al. Management of malignant pleural mesothelioma - part 3: Data from the Austrian Mesothelioma Interest Group (AMIG) database. Wien Klin Wochenschr 2016;128:627-34. [Crossref] [PubMed]
- Kristensen CA, Nottrup TJ, Berthelsen AK, et al. Pulmonary toxicity following IMRT after extrapleural pneumonectomy for malignant pleural mesothelioma. Radiother Oncol 2009;92:96-9. [Crossref] [PubMed]
- Opitz I, Friess M, Kestenholz P, et al. A New Prognostic Score Supporting Treatment Allocation for Multimodality Therapy for Malignant Pleural Mesothelioma: A Review of 12 Years' Experience. J Thorac Oncol 2015;10:1634-41. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [Crossref] [PubMed]
- Trousse DS, Avaro JP, D'Journo XB, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg 2009;36:759-63. [Crossref] [PubMed]


