
Predictors of pneumonitis-free survival following lung stereotactic body radiation therapy
Introduction
Stereotactic body radiation therapy (SBRT) is standard of care therapy for medically inoperable early stage non-small cell lung carcinoma and is often utilized in cases where non-operable management is preferred (1). SBRT for lung cancer is typically a well-tolerated treatment, largely owing to the reduced lung volumes exposed to high dose radiation and the sharp dose gradient of stereotactic treatments (2).
Radiation pneumonitis is the most common acute and long-term pulmonary toxicity (3). Its manifestation is a spectrum, from being radiographically detected changes to clinically apparent with symptoms of dyspnea, cough, and fever (3). The risk of clinical RP requiring medical intervention has been reported to be 9–28% after lung SBRT (4,5). Significant, i.e., grade 3 or higher, rates of RP have been reported typically in the low single digit range (6-11), but have been noted to be as high as 21% in a single series (12). Rare fatal cases of RP were reported in early experiences with SBRT for central and peripheral tumors alike (11,13).
Tumor motion during the respiratory cycle can affect the accuracy of treatment delivery; as a result, motion management is central to the safety and efficacy of lung SBRT. Respiratory gating, i.e., radiation delivery synchronized to a subset of the respiratory cycle, is one component of motion management. Restricting treatment to respiratory phases during which tumor motion is minimized can reduce the volume of normal lung parenchyma receiving ablative doses of radiation. Alternatively, patients can be treated using a free-breathing technique in which intra-fraction tumor motion is defined with the use of 4-dimensional computed tomography (4DCT).
The risk of radiation pneumonitis is thought to be related to the volume of irradiated lung. Various dosimetric and tumor-related factors have been explored as risk factors for radiation pneumonitis (4,8,14-16). Though no consensus exists on the most clinically appropriate dosimetric endpoint, mean lung dose has been the most commonly evaluated in numerous series (7-9,17,18). Similarly, the implementation of motion management techniques is heterogeneous (19). We aimed to explore techniques of motion management, in addition to patient and tumor-related characteristics, as predictors of radiation pneumonitis using a single institution multi-center approach.
Methods
Consecutive records for patients treated with lung SBRT at 4 clinical sites within a single academic institution were reviewed. An Institutional Review Board approved the retrospective collection of patient information and technical details of radiation therapy for patients treated with lung SBRT. For patients who had received more than a single lung SBRT course, only the first was included and analyzed for this study. Development of radiation pneumonitis was determined using all available records, which included multi-specialty follow-up visits with radiation oncologists, pulmonologists, medical oncologists, or thoracic surgeons, and, if applicable, hospitalizations. The earliest date of pneumonitis onset was recorded, and events were graded using the Common Terminology Criteria for Adverse Events Version 4.0. Other relevant demographic, tumor, and treatment characteristics including radiotherapy dose and fractionation, histology, size, location and centrality of the primary tumor were captured and maintained in a single database.
All patients were simulated with a 4DCT, allowing an estimate of tumor displacement at time of simulation. Selection for the method of motion management was determined based on the degree of tumor motion. Patients with ≥1 cm of maximum tumor motion were selected for respiratory-gated planning and delivery. The Real-time Position Management system (Varian Medical Systems, Palo Alto, CA) was used for phase-based gating. Otherwise, patients with a tolerable degree of intra-fraction tumor displacement were planned and subsequently treated using a free-breathing approach. Patients were immobilized using custom-made devices designed for SBRT, including CIVCO with abdominal compression or abdominal belt, or Medical Intelligence setups.
Descriptive statistics such as frequencies were generated for categorical variables; means and standard deviations were generated for numeric variables. Categorical patient and treatment characteristics were compared across motion management techniques using chi-squared tests or Fisher’s exact tests; numeric patient characteristics such as age were compared using ANOVA. Pneumonitis-free survival (PNFS) was defined as time from the date of first visit to earliest onset of radiation pneumonitis or last clinical follow-up, where those alive without pneumonitis were censored at last clinical follow-up date. The first pre-treatment visit typically precedes to the first fraction date by approximately 2 weeks.
Overall survival was defined as time from first visit date to date of death or last clinical follow-up. Local failure-free survival, regional failure-free survival, and distant metastasis-free survival were defined as time from first visit date to either date of local failure, regional failure, distant failure, or last clinical follow-up. For each endpoint, those not meeting the applicable endpoint were censored at last clinical follow-up date. Survival distributions were estimated using the Kaplan-Meier method and were compared using log-rank tests.
Univariate Cox proportional hazards models were fit for each covariate with each of the above endpoints. A multivariable Cox model was fit for PNFS as a function of motion management, gender, race, numeric age, T classification, number of fractions, dose, histology, smoking status, tumor centrality, and primary lobe of lung. Variables were subject to backwards elimination using an alpha of 0.2 for removal. Model assumptions were checked and verified. Firth’s penalized maximum likelihood estimation was used in the local control models due to a small number of local failures (n=12), in order to reduce bias in the parameter estimates and confidence intervals, and to handle empty cells (20,21). All analyses were performed using SAS 9.4 (Cary, NC); significance was assessed at the 0.05 level.
Results
Included for analysis were 208 patients, with a median follow-up of 23 months. A total of 208 lung SBRT courses representing the first treatment were selected from a database of 241 treatment courses to minimize the effect of multiple lung SBRT courses on the development of radiation pneumonitis and other clinical endpoints. The median age at time of treatment was 71 years (range, 39–95 years); 191 (91.8%) patients had early stage lung primaries (T1–2), with approximately half being T1a (45.6%). The majority of tumors were peripheral (62.6%), and the primary location was evenly distributed. 50 Gy in 5 fractions was the most commonly prescribed dose, followed by 48 Gy (12 Gy ×4, 22.6% of cases) and 54 Gy (18 Gy ×3, 17.3% of cases). Relatively few patients were prescribed either greater than 54 Gy or less than 48 Gy, most commonly 7.5 Gy ×8 and 34 Gy in a single fraction. Fifty-five (26.4%) patients were treated using respiratory gating, and 153 (73.6%) patients were treated free-breathing. Other relevant patient, tumor, and treatment characteristics are summarized in Table 1.
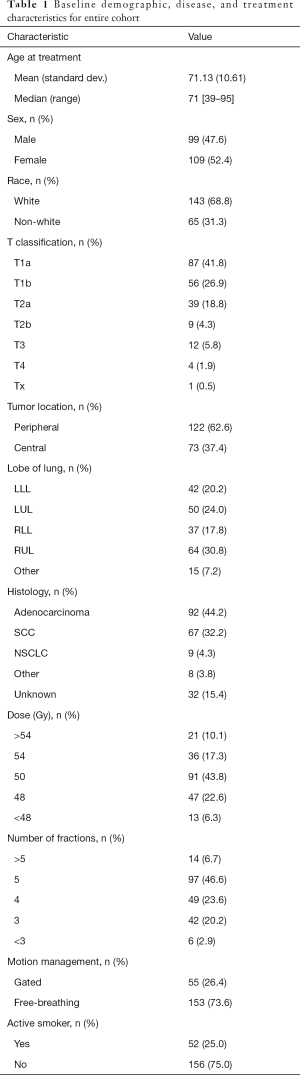
Full table
RP of any grade developed in 31.7% of patients overall. Of patients who developed RP, the majority of events were grade 2 or higher (62.1%); 31.8% were grade 3 or higher. Of all patients, 23.0% developed grade 2 or higher RP, and 10.1% developed grade 3 or higher radiation pneumonitis. Patients characteristics, with the exception of primary location and active smoking status, were balanced between motion management groups (Table 2). Patients being treated without respiratory gating were more likely to have upper lobe tumors compared to their gated counterparts (30.72% vs. 5.45% for left upper lobe; 37.25% vs. 12.73% for right upper lobe, P<0.001). A similar increase in the proportion of lower lobe tumors was seen in the gated group. Those treated free-breathing were more likely to be active smokers than those treated using respiratory gating (29.41% vs. 12.73%, P=0.014).
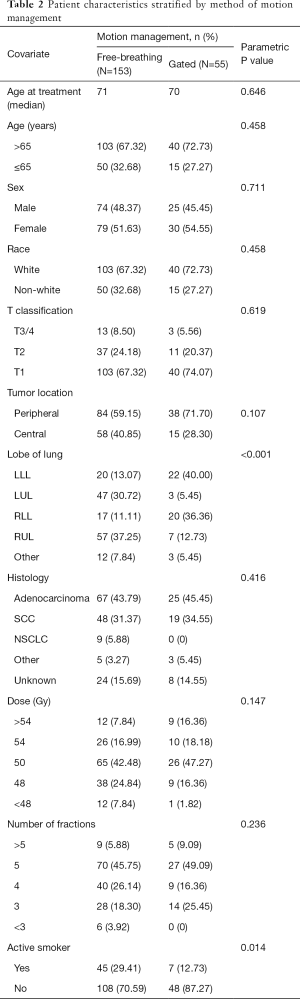
Full table
Patient, disease and treatment characteristics were assessed in the univariate setting for predictors of PNFS (Table 3). Peripherally-located lung tumors were associated with a reduced risk of RP [hazard ratio (HR) =0.43, log-rank P<0.001). Dose (log-rank P=0.002) and number of fractions (log-rank P=0.001) were both relevant to the risk of PNFS. Treatment to a dose of 48 Gy was associated with a longer interval of PNFS when compared to treatment to less than 48 Gy (HR =0.31, HR P=0.041); being treated to >54 Gy trended towards superior PNFS (HR =0.25, HR P=0.058). Treatment in 4 fractions was associated with superior PNFS (HR =0.21, HR P=0.021) compared to a course of fewer than 3 fractions. The 3- and >5-fraction courses both trended towards significantly longer PNFS intervals (HR =0.30, HR P=0.071 and HR =0.26, HR P=0.101 for 3- and >5-fractions, respectively). PNFS outcomes did not differ by motion management technique (log-rank P=0.383) (Figure 1). Neither primary tumor size, as approximated by T classification, nor lung lobe were significant for PNFS outcomes. Additionally, advanced age (>65 years), race, sex, or being an active smoker did not appear to impact the length of RP-free survival.
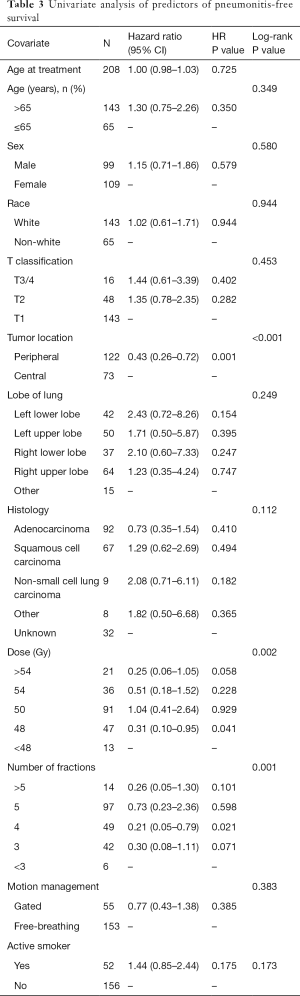
Full table
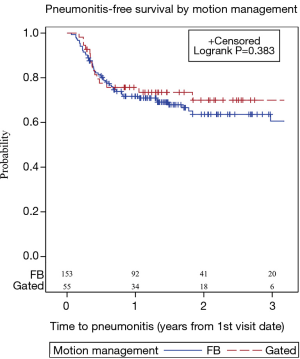
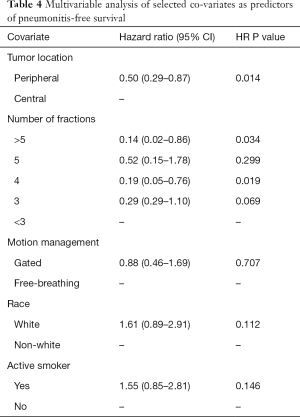
In the multivariable setting, peripheral tumor location was associated with superior PNFS over centrally-located tumors (HR =0.50, HR P=0.014, Table 4). Number of fractions remained significant for predicting PNFS. Receiving either a lung SBRT course in 4 or >5 fractions were associated with a longer RP-free survival interval (HR =0.19, HR P=0.019, and HR =0.14, HR P=0.034 for 4 and >5 fractions, respectively). A 3-fraction course trended towards longer PNFS (HR =0.29, HR P=0.069). Motion management technique did not appear to impact PNFS in the multivariable model (HR =0.88, HR P=0.707). No examined characteristic appeared to impact the length of local or regional failure-free survival. Characteristics associated with distant metastasis-free survival included age >65 years (HR =0.45, HR P=0.004) and histology (adenocarcinoma HR =1.49, squamous cell carcinoma HR =0.52, log-rank P=0.031).
Full table
Discussion
SBRT is a highly conformal form of ablative radiotherapy that has seen a pronounced rise in utilization over the past decade. Accurate treatment delivery is central to the concept of stereotactic treatments. Interest in managing tumor motion has paralleled improvements in image guidance. The optimal implementation and selection process of patients for respiratory gating are open questions in motion management.
The dosimetric advantages of gated treatment delivery in lung SBRT were detailed in an early report (22). Using 4DCT datasets, Underberg et al. found that the respiratory-gated planning treatment volume (PTV) was 33% of a conventional PTV derived from adding a 10 mm isotropic margin to the gross tumor volume (GTV). The amount of normal tissues encompassed by the 80% isodose volume in gated PTVs was 39% that of conventional PTVs. Notably, the reduction in target volume size was related to the magnitude of the 3D mobility vector such that patients with the most mobile tumors were most suitable for respiratory gating. Other dosimetric studies have corroborated these findings of smaller target volumes as well as reduced dose to organs at risk (23-25). How these dosimetric advantages translate into clinical outcomes is unclear.
This is one of the largest experiences analyzing patient, tumor, and treatment factors for the risk of radiation pneumonitis. The rate of radiation pneumonitis seen here is comparable to other reported SBRT series: 23.0% developed at least grade 2, and 10.1% grade 3 or higher RP. In the multivariate setting, both tumor location and number of fractions remained independent predictors for PNFS. We found patients who received 4-fraction and >5-fraction courses were associated with longer PNFS intervals. Interestingly, the subset of patients receiving 5-fraction SBRT did not appear to have superior PNFS (HR =0.52, P=0.299). A potential explanation is that 5-fraction courses are often used for centrally-located lesions. Treating tumors within 2 cm of the proximal bronchial tree can be associated with dramatically higher rates of toxicity, especially if no provisions are made to increase the total number of fractions (13,26,27).
There are several limitations to this study. A number of patient factors within this heterogeneous population were not included in the current analysis. Some of these characteristics may influence an individual’s risk of developing radiation pneumonitis, such as severity of pulmonary disease, indication for lung SBRT (medically inoperable versus patient preference), prior radiotherapy and whether respiratory gating was attempted but ultimately unable to be completed. Moreover, patients in this analysis were treated over a 7-year span, during which time other technical aspects of SBRT treatment likely evolved. Factors such as immobilization, image-guidance and planning techniques, and user experience, all of which can impact the efficacy and toxicity of treatment, were not accounted for in our analysis. Additionally, given its retrospective nature, patient selection at time of simulation for the type of motion management is another source of potential bias, limiting overall generalizability.
The optimal approach to motion management is uncertain; its practice is often a function of institutional approach, available technology, and clinical necessity. Practice patterns on the technical aspects of treatment planning and ways to manage respiratory motion vary among providers. Of over 100 thoracic radiation oncologists surveyed in the United States, 51% of respondents used abdominal compression, 31% used respiratory gating, 13% breath hold, and 29% employed multiple techniques to mitigate tumor motion. Likewise, there is no consensus on the maximum tolerated amount of tumor motion for non-gated treatment delivery. The American Association of Physicists in Medicine (AAPM) Task Group 76 report recommends the use of motion management strategies for tumor motion greater than 5 mm (28). With regard to patient selection, Korreman et al. and Guckenberger et al. have proposed using cutoffs >13 mm and >15 mm of tumor motion for respiratory gating, and >8 mm tumor motion for routine image guidance (29,30). These recommendations were based on measurements of tumor motion using 4DCTs and cone beam CTs, respectively.
Our institutional practice of using >10 mm of tumor motion as a threshold for gated planning appears to result in similar radiation-pneumonitis survival rates. In other terms, free-breathing planning and delivery using a standard ITV approach can be appropriate in patients with up to 1 cm of target lesion motion. Using this approach, our treatment groups stratified by gating are expected to be comparable target volume sizes and hence similar normal tissue dose to the lung parenchyma. In this study, tumor size as approximated by T classification was balanced between groups treated with either gated versus free-breathing techniques; more than 90% were T1 or T2. Patients with lower lobe tumors more commonly underwent gated treatment delivery, as expected given the greater degree of motion near the diaphragm (P<0.001).
Respiratory gating is an attractive way to moderate tumor motion, reduce uncertainty in localization, and potentially improve the therapeutic ratio of focal ablative radiation therapy. It is a non-invasive technique that the majority of patients are able tolerate with some coaching at the time of 4DCT simulation. There are, however, concerns that using an external signal can introduce positional uncertainty which can impact the fidelity of treatment delivery (31). As most commercially available gating systems use an external surrogate marker, its relationship with actual tumor motion is subject to change over time and between fractions. Gated treatments can also increase overall treatment time due to a lower duty cycle. Additionally, it requires more frequent imaging and a corresponding increase in radiation exposure. Finally, its quality assurance measures are labor-intensive (31).
Pulmonary disease is often a co-morbidity in SBRT lung patients who are medically inoperable, which provides a challenge to patient selection for motion management. Deep inspiratory breath hold is a another strategy to reduce intra-fraction motion; however as many as 50% of patients are unable to tolerate treatment with such setup (32). Irregular respiratory patterns can introduce delivery uncertainty, and up to 10% of patients may be excluded based on lack of reproducibility (30). There concerns were reflected in our series, as the free-breathing group had a significantly higher proportion of smokers compared to those who were treated with gating (29.41% vs. 12.73%, P=0.014).
Conclusions
PNFS did not differ between the patients treated with respiratory-gated and free-breathing lung SBRT. Centrally-located lesions and number of fractions were both independent predictors of radiation PNFS. As motion management techniques mature and are expected to become more sophisticated, careful patient selection and utilization of these strategies are necessary to optimize the therapeutic ratio.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: An Institutional Review Board approved the retrospective collection of patient information and technical details of radiation therapy for patients treated with lung SBRT.
References
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Roach MC, Videtic GMM, Bradley JD, et al. Treatment of Peripheral Non-Small Cell Lung Carcinoma with Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1261-7. [Crossref] [PubMed]
- Kang KH, Okoye CC, Patel RB, et al. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers (Basel) 2015;7:981-1004. [Crossref] [PubMed]
- Ong CL, Palma D, Verbakel WF, et al. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol 2010;97:431-6. [Crossref] [PubMed]
- Yamashita H, Takahashi W, Haga A, et al. Radiation pneumonitis after stereotactic radiation therapy for lung cancer. World J Radiol 2014;6:708-15. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013;85:190-5. [Crossref] [PubMed]
- Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012;82:457-62. [Crossref] [PubMed]
- Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009;91:307-13. [Crossref] [PubMed]
- Matsuo Y, Shibuya K, Nakamura M, et al. Dose--volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e545-9. [Crossref] [PubMed]
- Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2-5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007;2:21. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Kyas I, Hof H, Debus J, et al. Prediction of radiation-induced changes in the lung after stereotactic body radiation therapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;67:768-74. [Crossref] [PubMed]
- Tucker SL, Jin H, Wei X, et al. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:691-8. [Crossref] [PubMed]
- Valdes G, Solberg TD, Heskel M, et al. Using machine learning to predict radiation pneumonitis in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Phys Med Biol 2016;61:6105-20. [Crossref] [PubMed]
- Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010;97:65-70. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol 2009;48:571-7. [Crossref] [PubMed]
- Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol 2013;8:202-7. [Crossref] [PubMed]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27-38. [Crossref]
- Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001;57:114-9. [Crossref] [PubMed]
- Underberg RW, Lagerwaard FJ, Slotman BJ, et al. Benefit of respiration-gated stereotactic radiotherapy for stage I lung cancer: an analysis of 4DCT datasets. Int J Radiat Oncol Biol Phys 2005;62:554-60. [Crossref] [PubMed]
- Jang SS, Huh GJ, Park SY, et al. The impact of respiratory gating on lung dosimetry in stereotactic body radiotherapy for lung cancer. Phys Med 2014;30:682-9. [Crossref] [PubMed]
- Vlachaki M, Castellon I, Leite C, et al. Impact of respiratory gating using 4-dimensional computed tomography on the dosimetry of tumor and normal tissues in patients with thoracic malignancies. Am J Clin Oncol 2009;32:262-8. [Crossref] [PubMed]
- Xhaferllari I, Chen JZ, MacFarlane M, et al. Dosimetric planning study of respiratory-gated volumetric modulated arc therapy for early-stage lung cancer with stereotactic body radiation therapy. Pract Radiat Oncol 2015;5:156-61. [Crossref] [PubMed]
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89-93. [Crossref] [PubMed]
- Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874-900. [Crossref] [PubMed]
- Guckenberger M, Krieger T, Richter A, et al. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother Oncol 2009;91:288-95. [Crossref] [PubMed]
- Korreman S, Persson G, Nygaard D, et al. Respiration-correlated image guidance is the most important radiotherapy motion management strategy for most lung cancer patients. Int J Radiat Oncol Biol Phys 2012;83:1338-43. [Crossref] [PubMed]
- Cole AJ, Hanna GG, Jain S, et al. Motion management for radical radiotherapy in non-small cell lung cancer. Clin Oncol (R Coll Radiol) 2014;26:67-80. [Crossref] [PubMed]
- Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol 2004;14:65-75. [Crossref] [PubMed]


