
Interplay between immune cells in lung cancer: beyond T lymphocytes
Conclusive evidence has demonstrated that the immune system plays an instrumental role in preventing or promoting the development and progression of lung cancer. Progressively growing tumors escape immune control through a wide variety of mechanism which include: (I) secretion of immunosuppressive factors; (II) expression of immunosuppressive and anti-phagocytic molecules; (III) modulation of local and systemic metabolism; and (IV) recruitment and activation of host immunosuppressive cells that promote tolerance to tumors (1).
To date, most studies in the field of tumor immunology have focused on T cell-mediated adaptive immunity while the contribution of B-cells and humoral responses in cancer pathogenesis has largely been ignored. Therefore, the study by Wang and collaborators (2) represents an important contribution to the field. Using a syngeneic mouse model of lung cancer (C57BL/6J and C57BL/6 congenic CD45.1+ mice), the authors demonstrate that tumor-bearing mice exhibit an increase in the absolute number and percentage of myeloid-derived suppressor cells (Gr-1+CD11b+; MDSCs), both the granulocytic (CD11b+Ly6G+Ly6Clow) and the monocytic compartment (CD11b+Ly6G2Ly6Chigh). Furthermore, new and robust evidence is presented which supports the notion that during tumor progression, MDSCs modulate not only T-cell responses but also the number and function of specific B-cell subtypes via the IL-7 and STAT5 signaling axis. The aim of this editorial is to further discuss the results of the afore-mentioned study in the context of human lung cancer and to provide a conceptual framework for clinicians to more fully understand the relevance and implications of the study.
One of the advantages of the Lewis lung carcinoma (LLC) model is that tumor cells are immunologically compatible with the host into which they are implanted. Therefore, tumor engraftment was performed in immunocompetent mice, which allowed for immune-tumor interactions to be studied throughout the natural course of the disease. In earlier studies subcutaneous injection of LLC has been used as a relatively easy and reproducible method to evaluate subcutaneous tumor growth and lung metastasis. However, it has been acknowledged that due to microenvironment differences, subcutaneous implantation fails to accurately recapitulate the tumor biology of lung cancer or its response to therapy.
As a result, several methods have been developed to implant LLC cells directly into the lungs of mice (orthotopically). This can be achieved by either intrabronchial or intrathoracic injection into the pleural cavity or the lung parenchyma. Although intravenous and intracardiac injection of LLC cells (as performed in the study under discussion) leads to the rapid development of lung carcinoma in mice, it can be argued that this model is not strictly orthotopic and that it may in fact represent a process of rapid lung metastasis. It is unfortunate that neither ex vivo nor in vivo measurements of tumor mass in target organs was presented, since it leaves unanswered the question of whether the functional and phenotypical changes in B-cells populations correspond with tumor progression.
Although mechanistic experiments in murine models are essential to dissect the pathways by which B cells and humoral immunity contribute to lung cancer pathogenesis, it is important to keep in mind that immunological responses in murine models may not be transferable to humans due to intrinsic differences between species. For instance, whereas several studies have demonstrated that IL-7 activation of STAT5 is essential for the development of mouse B-cells and that disruption of this signaling axis results in the arrest of B-cell maturation at the pro-B stage (3), in humans, the regulatory role and timing of IL-7/IL-7Rα/STAT5 signaling during B-cell development remains a largely controversial topic. The classical model of human lymphopoiesis established that IL-7 is required for the proliferation of T-cell progenitors but not for the proliferation of B-cell progenitors. More recently, however, it has been reported that in humans, continuous pSTAT5 response to IL-7 is restricted to a rare population of B cell precursors, in which STAT5 phosphorylation can also be induced by TSLP (4). Furthermore, it has been shown that human B-cell production is increasingly dependent on IL-7Rα signalling which can be provided by IL-7 or TSLP (5). Together, these studies demonstrate that human B lymphopoiesis is affected by IL-7/IL-7Rα signaling, although perhaps to a lesser extent than in mice. This opens up the possibility that IL-7 may indeed affect anti-tumor immunity in humans by modulating not only T-cell but also B-cell responses.
However, there are conflicting reports regarding the effect of IL-7 on tumor growth. Whereas some studies have found that IL-7 can restore the number and function of CD8+ and CD4+ T-cells, others have found that it induces the expression of PD-1 and its ligands (6) and that IL-7Rα expression is associated with the immunosuppressive capacity of Tregs.
Similarly, there are studies showing that IL-7 signaling can prevent the apoptosis of human lung cancer cells, induce the epithelial-mesenchymal transition and metastasis of human breast and prostate cancer cells (7). In a large cohort of stage I lung adenocarcinoma patients, Suzuki et al. found that tumoral loss interleukin-12 receptor β2 (IL-12Rβ2) and IL-7R overexpression, as well as stromal FoxP3/CD3 ratio, are independent predictors of disease recurrence (8). In a subsequent study, the authors showed that ERα expression is another independent predictor of disease recurrence, which is associated with tumor IL-7R overexpression and FoxP3+ T cells infiltration (9).
Clinical trials using IL-7 as a vaccine adjuvant did not obtain promising results. For instance, in the first human dose-escalation trial, no objective responses were observed when recombinant IL-7 was administered in combination with two melanoma peptides (gp100 and MART-1). Similarly, there was no evidence of clinical activity in patients with non-hematologic malignancies treated with escalating doses of IL-7 or with a vaccine of melanoma cells engineered to express IL-7 (10)
These results are consistent with those of more recent studies showing that in patients with NSCLC, STAT5 expression and phosphorylation is associated with apoptosis inhibition, cell cycle progression, proliferation, invasion, and angiogenesis (11). Future studies could perhaps evaluate the expression and activity of cyclooxygenase-2 (COX-2), since it has been reported that STAT5 stimulates COX-2 expression in NSCLC. COX-2 is involved in the initiation and progress of tumors in situ and it is overexpressed in NSCLC, promoting angiogenesis and metastasis and inhibiting apoptosis (12).
The rationale behind assessing STAT5 in NSCLC is based on several reports describing that STAT-mediated pathways induce PD-L1 upregulation and contribute to tumor immune evasion. More specifically, it has been described that STAT3 recruits MDSC and it is related with decrease of immune cell infiltration in different tumors, including NSCLC. Moreover, the inhibition or silencing of STAT3 induces decrease expression of PD-L1 in EGFR-mutant and ALK-translocated cell lines (13). Thus, it has been proposed that STAT3 expression can be a potential biomarker of response to immunotherapy.
Myeloid-derived suppressor cells (MDSCs), a heterogeneous cell population of immature myeloid cells with highly immunosuppressive activity, have been shown to modulate various aspects of carcinogenesis including initiation, immune tolerance, progression, metastasis and therapy response. MDSC are comprised of early-stage MDSC (E-MDSC), immature mononuclear cells which are morphologically and phenotypically similar to monocytes (M-MDSC), and immature polymorphonuclear cells which, in turn, are morphologically and phenotypically similar to neutrophils (PMN-MDSC, formerly known as G-MDSC). These populations of MDSCs have become the focus of intense research in recent years (14). Indeed, the presence of different MDSCs populations both in the periphery and within the tumor, has been shown to correlate with poor clinical outcomes in patients with small and non-small-cell lung cancer (14-24). The results of these studies are summarized in Table 1. We have recently reported that survival is significantly reduced in NSCLC patients with a high percentage of PMN-MDSCs, particularly among patients with specific cytokine profiles (32,33).
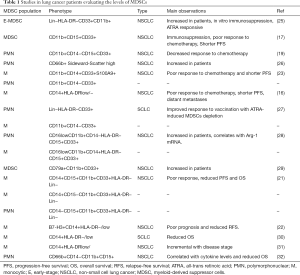
Full table
Collectively, the studies discussed thus far provide evidence indicating that comprehensive immunological profiles, that include a characterization of different populations of B-cells, necessary for the rational design and clinical development of novel immunotherapeutic agents. In particular, a large and growing body of literature indicates that agents targeting MDSCs are promising candidates to advance into subsequent stages of clinical development.
Acknowledgements
None.
Footnote
Conflicts of Interest: Oscar Arrieta has received honoraria as advisor, participated in speakers’ bureau and given expert opinions to Pfizer, AstraZeneca, Boehringer-Ingelheim, Roche, Lilly, and Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
References
- Yarchoan M, Johnson BA 3rd, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:209-22. [Crossref] [PubMed]
- Wang Y, Schafer CC, Hough KP, et al. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J Immunol 2018;201:278-95. [Crossref] [PubMed]
- Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol 2012;24:198-208. [Crossref] [PubMed]
- Bendall SC, Davis KL. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell 2014;157:714-25. [Crossref] [PubMed]
- Milford TA, Su RJ, Francis OL, et al. TSLP or IL-7 provide an IL-7Ralpha signal that is critical for human B lymphopoiesis. Eur J Immunol 2016;46:2155-61. [Crossref] [PubMed]
- Kinter AL, Godbout EJ, McNally JP, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol 2008;181:6738-46. [Crossref] [PubMed]
- Qu H, Zou Z, Pan Z, et al. IL-7/IL-7 receptor axis stimulates prostate cancer cell invasion and migration via AKT/NF-kappaB pathway. Int Immunopharmacol 2016;40:203-10. [Crossref] [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013;31:490-8. [Crossref] [PubMed]
- Kadota K, Eguchi T, Villena-Vargas J, et al. Nuclear estrogen receptor-alpha expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 2015;6:27505-18. [Crossref] [PubMed]
- Capitini CM, Fry TJ, Mackall CL. Cytokines as Adjuvants for Vaccine and Cellular Therapies for Cancer. Am J Immunol 2009;5:65-83. [Crossref] [PubMed]
- Pastuszak-Lewandoska D, Domanska-Senderowska D, Kordiak J, et al. Immunoexpression analysis of selected JAK/STAT pathway molecules in patients with non- small-cell lung cancer. Pol Arch Intern Med 2017;127:758-64. [PubMed]
- Pastuszak-Lewandoska D, Domanska D, Czarnecka KH, et al. Expression of STAT5, COX-2 and PIAS3 in correlation with NSCLC histhopathological features. PLoS One 2014;9:e104265. [Crossref] [PubMed]
- Attili I, Karachaliou N, Bonanno L, et al. STAT3 as a potential immunotherapy biomarker in oncogene-addicted non-small cell lung cancer. Ther Adv Med Oncol 2018;10:1758835918763744. [Crossref] [PubMed]
- Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [Crossref] [PubMed]
- Adah D, Hussain M, Qin L, et al. Implications of MDSCs-targeting in lung cancer chemo-immunotherapeutics. Pharmacol Res 2016;110:25-34. [Crossref] [PubMed]
- Huang A, Zhang B, Wang B, et al. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother 2013;62:1439-51. [Crossref] [PubMed]
- Srivastava MK, Bosch JJ, Thompson JA, et al. Lung cancer patients' CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother 2008;57:1493-504. [Crossref] [PubMed]
- Haverkamp JM, Smith AM, Weinlich R, et al. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity 2014;41:947-59. [Crossref] [PubMed]
- Liu CY, Wang YM, Wang CL, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136:35-45. [Crossref] [PubMed]
- Srivastava MK, Andersson A, Zhu L, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291-304. [Crossref] [PubMed]
- Vetsika EK, Koinis F, Gioulbasani M, et al. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res 2014;2014:659294. [Crossref] [PubMed]
- Zhang G, Huang H, Zhu Y, et al. A novel subset of B7-H3+CD14+HLA-DR-/low myeloid-derived suppressor cells are associated with progression of human NSCLC. Oncoimmunology 2015;4:e977164. [Crossref] [PubMed]
- Feng PH, Lee KY, Chang YL, et al. CD14+S100A9+Monocytic Myeloid-derived Suppressor Cells and Their Clinical Relevance in Non–Small Cell Lung Cancer. Am J Respir Crit Care Med 2012;186:1025-36. [Crossref] [PubMed]
- Solito S, Marigo I, Pinton L, et al. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014;1319:47-65. [Crossref] [PubMed]
- Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001;166:678-89. [Crossref] [PubMed]
- Brandau S, Trellakis S, Bruderek K, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 2011;89:311-7. [Crossref] [PubMed]
- Iclozan C, Antonia S, Chiappori A, et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013;62:909-18. [Crossref] [PubMed]
- Heuvers ME, Muskens F, Bezemer K, et al. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013;81:468-74. [Crossref] [PubMed]
- Luger D, Yang YA, Raviv A, et al. Expression of the B-cell receptor component CD79a on immature myeloid cells contributes to their tumor promoting effects. PLoS One 2013;8:e76115. [Crossref] [PubMed]
- Tian T, Gu X, Zhang B, et al. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark 2015;15:425-32. [Crossref] [PubMed]
- Liu J, Wang H, Yu Q, et al. Aberrant frequency of IL-10-producing B cells and its association with Treg and MDSC cells in Non Small Cell Lung Carcinoma patients. Hum Immunol 2016;77:84-9. [Crossref] [PubMed]
- Barrera L, Montes-Servin E, Hernandez-Martinez JM, et al. Levels of peripheral blood polymorphonuclear myeloid-derived suppressor cells and selected cytokines are potentially prognostic of disease progression for patients with non-small cell lung cancer. Cancer Immunol Immunother 2018;67:1393-406. [Crossref] [PubMed]
- Barrera L, Montes-Servin E, Barrera A, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol 2015;26:428-35. [Crossref] [PubMed]


