
The optimal treatment approaches for stage I small cell lung cancer
Introduction
Lung cancer remains the number one cause of cancer-related deaths in both men and women in the United States with over 220,000 new cases diagnosed annually (1). Out of these, small cell lung cancer (SCLC), a poorly differentiated neuroendocrine tumor, accounts for roughly 10–15% of cases (2). Most patients diagnosed with SCLC have a heavy smoking background and can present with respiratory symptoms or a variety of paraneoplastic syndromes including syndrome of inappropriate antidiuretic hormone secretion, Lambert-Eaton syndrome, or Cushing syndrome (3,4). A key feature of SCLC is its highly aggressive nature with a propensity towards early dissemination. Indeed, the incidence of detecting SCLC at an early stage is extremely low, with up to only 5% of patients presenting with clinical stage I disease after confirmation with mediastinal nodal sampling (5).
Historically, staging for SCLC was dichotomized as either “limited stage” (LS-SCLC) or “extensive stage” (ES-SCLC), the former categorization referring to any non-metastatic disease that could be safely treated within an acceptable, definitive radiation therapy (RT) plan (6). This unique two-stage system relative to other lung cancer types was established by the Veterans Administration Lung Study Group in 1957 and has governed clinical trial enrollment criteria and treatment algorithms which typically focus on the application of systemic therapy with or without local therapy (7). Over the past decade, however, studies showing survival differences by extent of primary and nodal disease have resulted in the adaptation of a more thorough classification system such as the American Joint Committee on Cancer (AJCC) TNM staging system for SCLC (8,9).
The current AJCC 8th edition staging system defines stage I SCLC as tumors ≤4 cm without nodal involvement (cT1–T2aN0) (6). Even for this optimal subgroup of patients, the 5-year overall survival (OS) remains poor at 31% despite intensive treatment (10,11). Local therapy in the form of primary resection for surgical candidates or definitive radiation therapy (RT) for inoperable patients is recommended by the National Comprehensive Cancer Network (NCCN) in addition to systemic therapy (6). However, optimal RT specifications such as the total dose and schedule are unknown and are the primary objectives of an ongoing clinical trial (12). More recently, the development and clinical application of stereotactic body radiotherapy (SBRT), a highly conformal RT modality capable of delivering ablative doses in 1 to 5 fractions, as part of the management of early stage lung cancers is also challenging current standards of care.
Operable clinical stage I SCLC
Although surgical resection with mediastinal lymph node dissection or sampling is the recommended form of local therapy for operable stage I SCLC, there have not been any prospective clinical trials examining the role of surgery for this group of patients. Interestingly, very limited randomized clinical trial data exists even for LS-SCLC of which the main findings do not support the use of surgery. The Medical Research Council phase III trial was one of the earliest trials that compared surgery versus radical RT for the management of SCLC of the bronchus. Out of 144 patients, 71 were randomized to surgery and exhibited a median OS of 6.5 months compared to 9.3 months in favor of RT (P=0.04) (13). The low survival outcomes observed in both arms is likely due to multiple factors including a higher risk population, lack of consistent chemotherapy usage (only 12% for all comers), the application of outdated RT techniques, and the fact that surgical patients underwent pneumonectomies which can be associated with higher perioperative morbidity and mortality rates compared to lobectomies (14,15).
The Lung Cancer Study Group 832 phase III randomized clinical trial, which investigated the role of surgery in operable SCLC patients who responded to chemotherapy, also found no survival benefit with surgery for LS-SCLC (median OS 15.4 vs. 18.6 months; P=0.78). However, this study was unique in that all randomized patients, including those treated with surgery, received thoracic RT and prophylactic cranial irradiation (PCI) (16). Challenges with extrapolating conclusions from these trials to subgroups of LS-SCLC patients are inevitable. A recent Cochrane analysis based on these two trials and another for all lung cancer histologies was inconclusive with respect to the role of surgery for clinical stage I SCLC (17). Despite the potentially outdated data and vastly heterogeneous patient population from the Lung Cancer Study Group 832 trial, a sub-analysis by TNM staging found improved survival with earlier stages of disease, including a median OS of 21.4 and 24.2 months for patients with clinical T1N0M0 and T2N0M0 SCLC, respectively. They also reported lower unresectability rates with T1 tumors at 6% compared to 16% for T2 and 40% for T3, thereby supporting the consideration and higher success rates of surgical resection for smaller tumors (16).
The majority of evidence in favor of surgery as a primary component of stage I SCLC treatment derives from institutional and large database retrospective studies. A series from Osaka, Japan evaluating all surgically treated SCLC from 1975 to 1994 found a median OS and 5-year OS of 53 months and 49% for clinical stage IA and 25 months and 47% for clinical stage IB patients, respectively (9). A more modern cohort exhibited continued survival improvements with a 5-year OS of 86% when treated with surgery followed by platinum-based chemotherapy (18). With regards to databases, the International Association for the Study of Lung Cancer database is one of the largest and contains clinicopathologic, treatment, and follow-up data for over 12,000 cases of SCLC from around the world (8). Of these, 349 completely resected cases were analyzed by TNM stage, and the 5-year OS was 44–53% for the 159 patients with pathologic stage I disease (19). Moreover, the National Cancer Data Base (NCDB), which captures over 70% of all newly diagnosed cancer cases in the United States, was also queried to show improved survival for early stage disease after surgery compared to nonsurgical regimens (20). Table 1 summarizes the randomized trials and several retrospective studies that have led to the general consensus of adopting surgery as a part of multidisciplinary care for patients with operable stage I SCLC.
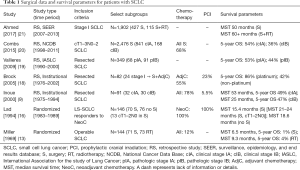
Full table
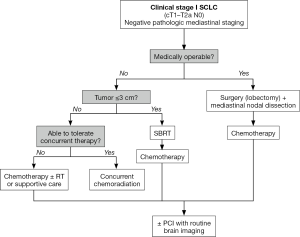
When considering a patient for curative surgery, it is important to emphasize several points in addition to a thorough functional assessment. Firstly, it is critical that proper clinical staging be performed. Given the tendency of SCLC to spread early, occult nodal metastatic disease in the setting of negative clinical nodal involvement by CT imaging is of significant concern. Inoue et al. reported a staging accuracy by CT scans of 68% with an underestimation of the N factor in 31% (N=19) of SCLC patients (9). Therefore, clinicians should only offer surgical interventions to patients in which a negative nodal involvement has been confirmed by either endoscopic ultrasonographic or mediastinal staging procedure (6,21). Moreover, since stage I tumors should be 4 cm or less in greatest dimension, managing a complete resection via a lobectomy should be achievable for the majority of patients as sublobar resections and pneumonectomies can result in worse perioperative and long-term outcomes (20). Complete resection by no means absolves the need for chemotherapy to address a systemic disease diagnosed at an early stage. Cisplatin and etoposide (EP), typically given over the course of 4 to 6 cycles, is considered modern, first-line systemic therapy for SCLC regardless of stage (6,22). Any patient with residual or pathologically node positive disease should be offered adjuvant chemoradiation, delivered concurrently or sequentially (6) (Figure 1). Lastly, clinicians should be mindful of healthcare disparities that exist in our society in order to actively attempt to minimize their influence on treatment selection for patients. For example, one study found that African Americans were approximately 50% less likely to undergo surgery than white patients (OR =0.49, P=0.001) and that lower rates of surgical resection were performed in elderly patients and Medicaid recipients (23).
Medically inoperable stage I SCLC
As previously mentioned, systemic therapy, ideally delivered concurrently with thoracic RT, has been established as the main stay treatment algorithm for LS-SCLC, including subsets of patients who have been found to have pathologic node-positive disease on mediastinal sampling or those with stage I disease that are deemed poor surgical candidates (6). The latter group is not trivial, as SCLC patients generally have greater smoking history and comorbidities than their NSCLC counterparts.
Conventionally fractionated RT
Two landmark analyses were published in 1992, one of which analyzed data from 2,140 LS-SCLC patients enrolled on 16 randomized clinical trials. This study found a 5.4% OS benefit at 3 years along with a local control improvement from 16% to 34% with the addition of thoracic RT to chemotherapy (24,25). Critiques of these trials centered on the heterogeneity of patients and the RT plans as well as the use of outdated cyclophosphamide or doxorubicin-based chemotherapy regimens. It was not until the results of the Intergroup 0096 phase III randomized trial were reported that a safe and acceptable RT scheme was nationally adopted. In this trial, a total of 381 patients were randomized from 1989 to 1992 to receive either twice-daily (BID) or once-daily thoracic RT to a total dose of 45 Gy delivered at 1.5 Gy/fraction over 3 weeks or 1.8 Gy/fraction over 5 weeks, respectively, concurrently with 4 cycles of EP27. Despite the rates of grade 3 esophagitis being higher with the BID regimen (27% vs. 11%; P<0.001), 5-year OS was significantly improved by 10% with BID fractionation (26% vs. 16%) (26). These findings are likely associated with the notion that the dose-response curve for SCLC lacks a shoulder, thereby representing a potential biological advantage of BID treatments over daily treatments for more optimal tumor killing.
However, the inferior biologically equivalent radiation dose with the once-daily RT arm from the INT 0096 study has led to continued investigations for the optimal thoracic RT schema. The CONVERT trial, which was a phase III, randomized, superiority trial comparing standard 45 Gy (BID) in 3 weeks to 66 Gy in 33 fractions (QD), found equivalent survival outcomes at 2 years between its two RT arms (27). The CALGB 30610/RTOG 0538 is an ongoing phase III trial that will hopefully yield relevant, randomized data on 45 Gy (BID) versus a higher QD fractionation scheme of 70 Gy at 2 Gy/fraction over 7 weeks (12). Besides RT dose and fractionation variations, several studies have also demonstrated improvements in survival when initiating RT early during the first 1–2 cycles of chemotherapy and when completing RT in 30 days or less (28-30). All previously described studies in LS-SCLC have laid the foundation for present day national guidelines; however, it is arguable that patients with very limited disease may be at a disadvantage by applying the same treatment algorithms to those with more extensive disease burden. Moreover, as further advancements in treatment modalities emerge, established guidelines should be re-examined, particularly for stage I SCLC patients who have the best prognosis with respect to tumor characteristics and may therefore benefit more from aggressive local therapy.
The application of SBRT for stage I SCLC
SBRT, otherwise known as stereotactic ablative radiotherapy (SABR), is a technique that relies on rigid immobilization and superior image guidance relative to conventional radiotherapy in order to deliver highly conformal, ablative radiation doses in 5 fractions or less to a designated site of disease. In recent years, there has been a burgeoning interest in evaluating the role of SBRT for inoperable stage I SCLC, a movement largely influenced by mirrored questions being answered in the realm of early stage NSCLC. It is known that patients with stage I NSCLC who are treated with conventional radiotherapy alone can have suboptimal local control rates of 30–50% and tend to experience inferior survival compared to those who are treated surgically (31). The potential biological advantages of delivering ablative, curative doses via SBRT was therefore investigated in several trials including the phase II RTOG 0236 trial which treated 55 inoperable patients with T1–2 (≤5 cm) N0 NSCLC tumors to 54 Gy in 3 fractions. The 3-year OS was promising at 56% with an associated excellent primary tumor control rate of 98% and locoregional control rate of 87% (32). Subsequent SBRT studies have demonstrated the importance of achieving a calculated biologic effective dose of 100 Gy or greater and have outlined guidelines for treating centrally located tumors (33,34). Finally, a pooled analysis of two discontinued phase III randomized trials comparing SBRT to surgery in operable patients also supports SBRT as an acceptable alternative to surgery with excellent 3-year OS of 95% (vs. 79%) and limited treatment morbidity (32,35-37).
As SCLC is thought to be more radiosensitive than NSCLC, the concept of SBRT as an ideal form of local therapy for stage I SCLC is rather logical but has yet to be tested in a randomized setting. Table 2 summarizes several retrospective SBRT series for early stage SCLC. Most case series are small with a less than 10 patient experience. The Kyushu University Hospital, for example, published its experience with 8 patients treated with SBRT to 48 Gy in 4 fractions and found a 3-year OS and DFS of 72% and 86%, respectively. Moreover, none of their patients developed local progression (41). From 2004 to 2010, 6 medically inoperable stage I SCLC patients treated with various SBRT fractionation schemes at another institution had a 100% local control rate with an observed 1-year OS of 63% (42). Despite the relatively small sample sizes, all institutional series have also reported minimal toxicity with SBRT, which is a key advantage over conventionally fractionated RT since most SCLC patients are past smokers and may have multiple cardiac and pulmonary comorbidities.
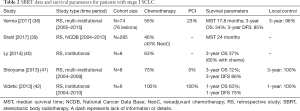
Full table
Verma et al. recently published the largest series of SBRT cases, which included 74 patients (76 treated lesions) with stage I SCLC treated at over 24 different institutions. Ninety-six percent of the patients received SBRT with a BED ≥100 Gy. The median age was 72 years, median tumor size was 2.5 cm, and chemotherapy and PCI use occurred in 56% and 23% of cases, respectively. Median OS was 18 months for the entire cohort and the 3-year local control rate was 96% (38). Given the findings of this study, several conclusions are noteworthy to mention. Firstly, although SBRT can result in excellent primary tumor control equivalent to rates found in NSCLC series, the associated 3-year OS of 34% is rather low compared to inoperable NSCLC patients. This likely reflects the propensity of SCLC to metastasize quickly even when diagnosed at early stages of disease, the need for proper clinical staging (only 19 patients underwent mediastinal sampling), and the importance of systemic therapy (46% of recurrences were distant). It is unknown why almost half of these patients did not receive chemotherapy; however, the receipt of chemotherapy was found to improve OS [hazard ratio (HR) =0.41; 95% confidence interval (CI), 0.21–0.80; P=0.1] and DFS (HR =0.37; 95% CI, 0.17–0.82; P=0.008) (38). Therefore, we strongly recommend against exempting stage I SCLC patients from first-line chemotherapy if the patient can tolerate the treatment. Lastly, tumor size should be factored into a clinician’s consideration of SBRT for patients, as inoperable disease with tumors measuring ≥2 cm were found to have significantly worse outcomes compared to smaller tumors (HR =2.8; 95% CI, 1.32–5.94; P=0.008) (38). Overall, greater efforts should be made to establish the role of SBRT for stage I SCLC as the use of SBRT is on the rise, with one NCDB study reporting 6.4% of cT1–2N0 SCLC patients being treated with SBRT in 2013 compared to 0.4% in 2004 (39).
The role of prophylactic cranial irradiation in early stage SCLC
PCI is controversial for stage I SCLC. Historically, PCI was investigated in randomized clinical trials for both LS-SCLC and ES-SCLC disease given the observation that over 50% of patients developed metastatic lesions to the brain. Aupérin et al. performed a meta-analysis capturing data from 7 trials comparing PCI versus none for 987 patients (86% LS-SCLC) who underwent complete remission after initial therapy. The relative risk of death after PCI was 0.84, corresponding to a 5.4% survival benefit at 3 years (21% vs. 15%). PCI also increased the rate of DFS while significantly reducing the cumulative risk of intracranial metastasis by 25% (43). Therefore, for LS-SCLC patients who undergo a complete or partial response, PCI delivered in a preferred dosing of 25 Gy in 10 fractions, is a category 1 recommendation in current national guidelines (6,44).
PCI is not without its potentially debilitating sequelae. Whole brain radiation therapy for the management of any metastatic brain disease can lead to significant chronic neurotoxicity including cognitive decline, thereby raising concerns with regards to its usage in elderly patients or those with poor performance status or active neurological deficits (45,46). Recent studies have shown that the risk of developing brain metastasis in stage I SCLC is lower than for all other stages, ranging from 10–15% (38,47-49). In the modern era of advanced imaging, delaying radiation to the brain by performing routine surveillance magnetic resonance imaging every 3–4 months within the first 2 years is an appealing alternative to PCI. One study examining factors influencing the utilization of PCI for LS-SCLC found that the most common reason for PCI omission was patient refusal due to neurotoxicity concerns (38%) (50). Hippocampal sparing PCI to reduce the risks of neurocognitive impairment after PCI is being investigated for patients with LS-SCLC. However, existing data in literature to support its use is limited and one small prospective study raised concerns of potential risks of failures in the spared region of the brain after hippocampal-sparing PCI (51,52). A retrospective review of 349 completely resected SCLC patients treated at Shanghai Chest Hospital between 2006 and 2014 found that only one-third of patients were treated with PCI out of which only pathologic stages II and III exhibited a significant survival benefit with PCI (53).
Therefore, the benefit of PCI in this subpopulation of SCLC remains inconclusive. Instead, a thorough discussion between clinicians and patients weighing the therapeutic benefits and adverse effects of PCI is advised. Another valid consideration is whether the patient has the resources or is compliant enough to undergo close surveillance instead of PCI. If PCI is recommended, memantine, an N-methyl-D-aspartate receptor antagonist, should be given concurrently as a recent trial demonstrated a significantly prolonged the time to cognitive decline after its use (54).
Conclusions
Clinical stage I SCLC represents a unique and small population of patients with lung cancer. Although they present with optimal tumor characteristics, the aggressive nature of their disease makes it essential to continually consider all multimodal therapy options, including current and evolving ones. Our general treatment recommendations when assessing a patient with stage I SCLC are outlined in Figure 1. A multidisciplinary evaluation with pathologic mediastinal staging is advised. For surgical candidates, complete resection of their disease followed by adjuvant chemotherapy is currently considered standard of care. In medically operable patients, lack of contemporary randomized data in this subpopulation of LS-SCLC patients leaves room for improvement on understanding the most ideal treatment algorithm. Current national guidelines recommend systemic therapy with thoracic radiotherapy if feasible. However, the utilization of SBRT as a form of local therapy for inoperable patients is on the rise and should be further investigated in randomized clinical trials. After a thorough review of several institutional SBRT series, patients with tumors ≤3 cm are likely ideal candidates for SBRT, with consideration of SBRT in patients with larger, node-negative tumors in select cases. Regardless of chosen local therapy modality, systemic therapy should always be considered the backbone of treatment as it addresses the tendency of SCLC to metastasize early. The benefit of PCI is less clear for stage I SCLC and should be offered concurrently with memantine only after a thorough discussion with the patient regarding potential neurotoxicities. Routine surveillance with serial brain imaging is an acceptable alternative to PCI.
Acknowledgements
None.
Footnote
Conflicts of Interest: Grant support: Genentech, Inc., New River Labs, Beyond Spring Pharmaceuticals, Hitachi Chemical Diagnostics. Advisory Board: AstraZeneca, Inc.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064-72. [Crossref] [PubMed]
- Payne M, Bradbury P, Lang B, et al. Prospective study into the incidence of Lambert Eaton myasthenic syndrome in small cell lung cancer. J Thorac Oncol 2010;5:34-8. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Kalemkerian GP, Loo BW, Chair V, et al. NCCN Guidelines Version 2. 2018 Panel Members Small Cell Lung Cancer Charles Florsheim Patient Advocate. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed March 4, 2018.
- Puglisi M, Dolly S, Faria A, et al. Treatment options for small cell lung cancer - do we have more choice? Br J Cancer 2010;102:629-38. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615-9. [Crossref] [PubMed]
- Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM Edition. J Thorac Oncol 2009;4:300-10. [Crossref] [PubMed]
- Small Cell Lung Cancer Survival Rates, by Stage. Available online: https://www.cancer.org/cancer/small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed March 4, 2018.
- Phase III Comparison of Thoracic Radiotherapy Regimens in Patients with Limited Small Cell Lung Cancer Also Receiving Cisplatin and Etoposide. Available online: https://www.rtog.org/LinkClick.aspx?fileticket=HS82MX12XxY%3D&tabid=331. Accessed March 15, 2018.
- Miller AB, Fox W, Tall R. Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet 1969;2:501-5. [Crossref] [PubMed]
- Strand TE, Rostad H, Damhuis RA, et al. Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude. Thorax 2007;62:991-7. [Crossref] [PubMed]
- Lee ES, Park SI, Kim YH, et al. Comparison of Operative Mortality and Complications between Bronchoplastic Lobectomy and Pneumonectomy in Lung Cancer Patients. J Korean Med Sci 2007;22:43. [Crossref] [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-3S. [Crossref] [PubMed]
- Barnes H, See K, Barnett S, et al. Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst Rev 2017;4:CD011917. [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the Case for Lobectomy in Stages I, II, and IIIA Small-Cell Lung Cancer Using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Ahmed Z, Kujtan L, Kennedy KF, et al. Disparities in the Management of Patients With Stage I Small Cell Lung Carcinoma (SCLC): A Surveillance, Epidemiology and End Results (SEER) Analysis. Clin Lung Cancer 2017;18:e315-25. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665-72. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [Crossref] [PubMed]
- Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [Crossref] [PubMed]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. [Crossref] [PubMed]
- Fried DB, Morris DE, Poole C, et al. Systematic Review Evaluating the Timing of Thoracic Radiation Therapy in Combined Modality Therapy for Limited-Stage Small-Cell Lung Cancer. J Clin Oncol 2004;22:4837-45. [Crossref] [PubMed]
- Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336-44. [Crossref] [PubMed]
- Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: A systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 2007;33:461-73. [Crossref] [PubMed]
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Maquilan G, Timmerman R. Stereotactic Body Radiation Therapy for Early-Stage Lung Cancer. Cancer J 2016;22:274-9. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Zhen W, et al. Stereotactic Radiotherapy for Stage I Small Cell Lung Cancer. Oncologist 2016;21:131-3. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Allen PK, et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:362-71. [Crossref] [PubMed]
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer 2017;103:11-6. [Crossref] [PubMed]
- Ly NB, Allen PK, Lin SH. Stereotactic body radiation therapy for stage I small cell lung cancer: a single institutional case series and review of the literature. J Radiat Oncol 2014;3:285-91. [Crossref]
- Shioyama Y, Nakamura K, Sasaki T, et al. Clinical results of stereotactic body radiotherapy for Stage I small-cell lung cancer: a single institutional experience. J Radiat Res 2013;54:108-12. [Crossref] [PubMed]
- Videtic GM, Stephans KL, Woody NM, et al. Stereotactic body radiation therapy-based treatment model for stage I medically inoperable small cell lung cancer. Pract Radiat Oncol 2013;3:301-6. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Wolfson AH, Bae K, Komaki R, et al. Primary Analysis of a Phase II Randomized Trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of Different Total Doses and Schedules of Prophylactic Cranial Irradiation on Chronic Neurotoxicity and Quality of Life for Patients With Limited-Disease Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2011;81:77-84. [Crossref] [PubMed]
- Wilke C, Grosshans D, Duman J, et al. Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol 2018;20:597-607. [Crossref] [PubMed]
- Le Péchoux C, Laplanche A, Faivre-Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann Oncol 2011;22:1154-63. [Crossref] [PubMed]
- Wu AJ, Gillis A, Foster A, et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol 2017;125:130-5. [Crossref] [PubMed]
- Bloom BC, Augustyn A, Sepesi B, et al. Prophylactic Cranial Irradiation Following Surgical Resection of Early-Stage Small-Cell Lung Cancer: A Review of the Literature. Front Oncol 2017;7:228. [Crossref] [PubMed]
- Halthore A, Goenka A, Sharma R, et al. Prophylactic Cranial Irradiation for Resectable Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:115-9. [Crossref] [PubMed]
- Lok BH, Ma J, Foster A, et al. Factors influencing the utilization of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Adv Radiat Oncol 2017;2:548-54. [Crossref] [PubMed]
- Redmond KJ, Hales RK, Anderson-Keightly H, et al. Prospective Study of Hippocampal-Sparing Prophylactic Cranial Irradiation in Limited-Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:603-11. [Crossref] [PubMed]
- Kundapur V, Ellchuk T, Ahmed S, et al. Risk of Hippocampal Metastases in Small Cell Lung Cancer Patients at Presentation and After Cranial Irradiation: A Safety Profile Study for Hippocampal Sparing During Prophylactic or Therapeutic Cranial Irradiation. Int J Radiat Oncol Biol Phys 2015;91:781-6. [Crossref] [PubMed]
- Xu J, Yang H, Fu X, et al. Prophylactic Cranial Irradiation for Patients with Surgically Resected Small Cell Lung Cancer. J Thorac Oncol 2017;12:347-53. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]


