
Implementation of lung cancer screening at the national level: Polish example
Introductory remarks
Despite the ongoing effort including implementation of multiple regimes in clinical oncology and thoracic surgery, the poor outcome of lung cancer treatment resulting, at best, in average 5-year survival of 15%, has remained virtually unchanged throughout the last 3 decades (1). Any preventive action, either primary or secondary, tends to be less expensive than treatment of advanced diseases, provided that it is appropriately designed and targets a population at risk. In contrast to basic sciences, in which the invested resources rarely are related to economic gain, medical prevention always entails the economic return. It has recently been demonstrated by an actuarial analysis performed in the US (2). Since this sort of analysis strongly relies on the local conditions including economic considerations, social and healthcare policy, the actuarial results should not be automatically transferred between countries.
Current situation of population based screening programs
In the USA, the American College of Radiology Imaging Network, set up the large-scale National Lung Screening Trial (NLST). This trial was terminated in 2010 and the results were published in 2011 (3). The primary end-point of the study was to assess lung cancer mortality. High risk patients aged 55 to 74-year-old with at least 30 pack-years’ smoking history, were randomized to undergo annual low-dose CT.
The rate of death from lung cancer was relatively reduced by 20% (95% CI: 6.8–26.7%; P=0.004) in the low-dose CT group (247 versus 309 per 100,000 person-years). The overall death rate was reduced in the low-dose CT group by 6.7% (95% CI: 1.2–13.6%; P=0.02).
The European randomized trial NELSON (acronym from Dutch: Dutch-Belgian Lung Cancer Screening Trial) accrued between 2004 and 2006 a total of 15,822 participants, aged 50–75 having smoking history of 15 pack-years or more. They were randomly assigned to the low-dose CT group or the control group (no screen). It was estimated that with this size of sample, a 25% mortality reduction could be demonstrated 10 years after randomization (4).
The final results of NELSON trial has not been published in a peer-reviewed journal yet. Quite recently, at the end of 2018 the NELSON researchers announced the data analysis of 10-year period since implementing the study. About 50% of the cancers diagnosed in the screening arm were early stage, and 65% to 70% were stages IA to II; about 70% of cancers in the control arm were stage III/IV at diagnosis.
Overall, CT scanning decreased mortality by 26% in high-risk men and up to 61% in high-risk women over a 10-year period (http://www.ascopost.com/issues/october-25-2018/nelson-trial/). Since the NELSON trial has been carried out on European population it is hoped that it will motivate EU countries to start a preparing LDCT-based lung cancer screening programs.
European scientific societies, such as the European Society of Radiology (ESR), the European Respiratory Society (ERS) and the European Society of Thoracic Surgery (ESTS) recommend screening as part of long-term programs conducted in comprehensively equipped, multidisciplinary and certified centers (5,6).
At the very beginning of preparation to construct a nationwide LDCT screening program, one should modify the environment of medical professionals. First of all local programs should be launched to give physicians and other medical staff the opportunity to deepen the knowledge pertaining to screening. Once they are engaged in the process of patients’ recruitment, screening methodology, data assessment and capable of managing participants individually, the experience may efficiently be disseminated. Within the years, provided the motivation and persistence of the initiators is secured, LDCT lung cancer screening may become to be perceived as a routine medical regimen. It is worth to stress the process of absorbing lung cancer screening is not necessarily guided by the accumulated scientific data. Apparently, the barriers in the medical professional community are rooted in the conservative attitude to a novel regimen, which, on the other hand, is justified as a measure to ensure safety to patients. In Poland, it has taken 10 years from launching local lung cancer screening programs in Szczecin, Warsaw, Gdansk and Poznan, funded from distinct resources, until the readiness to initiate a program covering the whole country. The successful debate, which gathered clinicians of many specialties, patient advocacy organizations and policy makers took place two and a half years ago under auspices of the Health Committee of the Senate of the Republic of Poland. Since then we have managed to propose the multidisciplinary consensus statement on LDCT lung cancer screening (7) and to create the governmental funding based nationwide program, which has been approved by a regulatory body, i.e., the Agency of Medical Technology Assessment.
Risk assessment modelling
Lung cancer risk prediction, which seeks to target the group who benefits most from the screening, constitutes a very complex task. At the same time the role of precise risk modelling is difficult to overestimate since it is directly related to the allocation of financial resources, which are usually limited. Owing to financial reasons and a strong association of the malignancy with smoking, the program for the detection of early lung cancer does not cover the entire population, but only at risk subpopulation defined by specific age and tobacco exposure (3).
After re-analysis of the NLST and PLCO cohorts, it is recommended to use predictive models of individual risk of developing lung cancer. They are based on additional risk factors, that allow to target more precisely the screening population (5,8). Nevertheless, determining the target population of lung cancer screening programs remains a complex and empirical problem related to the fact that the occurrence of lung cancer in a particular person is a multidimensional variable, similar to the trajectory of health. The predictive model appropriate for the American population not necessarily is appropriate for the Polish population because of heterogeneity and dissimilar response to various factors that are determined by environmental influence.
In the past two decades, considerable progress has been made in the modelling of the risk group screened with LDCT and in the interpretation of CT scans. This allows to reduce the number and frequency of examinations in the program and confines unnecessary diagnostic procedures.
There are several methods employed to reduce the number of false positive results and thus improving an appropriate population targeting. The use of linear regression equations that include the epidemiologic elements and socioeconomic data, facilitates to estimate the individual risk of developing lung cancer resulting from the characteristics of the population of concern (7,9). Only three of many prediction models have provided a more accurate selection of individuals for screening, compared to the NLST criteria.
Furthermore, models that take into account the patient’s clinical details and the morphological features of the nodule, can determine the degree of probability of the nodule being malignant (7). Both classes of the prediction modeling add up in individual qualification of subjects for inclusion in a screening program (shared decision-making), particularly upon discussion of the benefits and side effects of the program procedures being planned.
Therefore, the initial scenario is A-55-74-20-15, with a reduction of age in the case of an additional risk factor in accordance to the National Comprehensive Cancer Network (NCCN) recommendations. Then, the screening scenario of the target population takes the form of A-50-74-20-15. The scenario consecutive segments signify: a-annual, age of commencing and terminating screening, number of pack years and the maximal number of years since quitting.
Cautious estimates indicate that the number of people who meet the inclusion criteria for screening amounts to around 2 million in Poland.
Radiological requirements and protocols
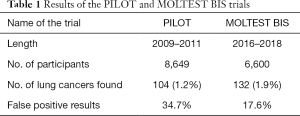
High-volume centers with radiologists experienced in reading LDCT images have a higher cancer detection rate and accuracy in the screening programs (10-12). However, there are no specified recommendations regarding the accreditation of radiologists participating in the majority of such programs. In USA American College of Radiology (ACR), set the limit of 300 chest CT scans over 36 months for those who want to apply for the accreditation. In practice false positive rate in lung cancer screening is a derivative of radiologists experience that has been proven by the comparison of two consecutive screening programs performed in Gdańsk (13). The experience gained by the radiologists participating in first PILOT program between 2009–2011 resulted in a significant, double fold reduction of interval LDCT examinations in MOLTEST BIS study performed between 2016 and 2018 (Table 1). Authors highlighting the difficulty in assessing different features of lung nodules in LDCT lung cancer screening and the lack of satisfactory agreement between radiologists (14,15). LDCT images are difficult to read (16), as different nodules require various approaches and instruments for their assessment. The management of positive results is a complex process. The nodules’ measurements should be conducted using only identical, certified methods, otherwise the results might be false, and as a result a wrong clinical decision can be undertaken (17).
Full table
It supports the idea that to achieve the highest quality all radiological examinations in the national program should be evaluated in one experienced center, which would be beneficial due to the higher quality of testing, as well as the simplification of surveillance procedures, which would be beneficial radiation therapy. However, it would be difficult to review all the examinations carried out in all screenings in the country of the size of Poland. That’s why we decided to perform central reading of CT images on a regional scale, i.e., 2 to 4 centers in Poland. So the main area of concern relates to the provision of screening services. The most effective approach is to centralise these services, and to supervise regional centres (Table 2).

Full table
The assessment of coronary artery calcification should be performed as the added value measurement. Emphysema assessment can contribute as an additional risk factor of lung cancer.
Equipment
American College of Radiology-Society of Thoracic Radiology (ACR-SR) has developed the minimal technical standards for CT machines participating in lung cancer screening in US. Spiral scanners with a minimum of 16 rows and lowest possible radiation dose are accepted, and follow up of the “As Low As Reasonably Achievable” (ALARA) should be followed.
For an average-sized patient, the maximum accepted value of kV is 100–140 mAs should be set in combination with kVp, to meet the computed tomography dose index (CTDIvol) ≤3 mGy using the 32 cm diameter CTDI phantom. CTDIvol should not exceed 3 mGy, ranged between 2.5–6 mGy in the NLST and NELSON trials, with an effective dose of 1 mSv in men and 1.3 mSv in women (18). The low-voltage CT scans (80 kV) using the full iterative reconstruction algorithms allow for an adequate assessment of the size and density of lung nodules in cancer patients (19) and a reduction of the effective dose to 0.1 mSV (20). Maximum tube rotation time should be ≤0.5 seconds and pitch—between 0.7 and 1.5. The preferred value for reconstructed image width is ≤1 mm and the permissible value is ≤2.5 mm. Iterative image reconstruction algorithms with variances of model-based iterative reconstruction (MBIR) are recommended, as they can influence the improvement of image quality and reduce patient dose by as much as 80–90 percent for the same type of CT scans. To assess the risk associated with the impact of ionizing radiation, the quantification, monitoring and reporting of the radiation dose is mandatory (16). LDCT and PET/CT scans in a 10-year COSMOS study observation indicate that the additional risk of induced malignancies is 0.05% (21).
Nodule management protocol
Nodules should be categorised into two different groups according to their density (Table 3). Currently, two systems for assessing lung nodules are recommended in low dose CT studies: Lung-RADS and the system proposed by European position statement (EUPS) (22). According to the Lung-RADS criteria, a positive result in LDCT imaging is a solid or partially solid nodule with an average dimension of not less than 6 mm. With such a cut-off value for lung nodules, the retrospective assessment of false positive rates in NLST would be 10.6% according to McKee and 12.8% according to Pinsky (8,23). Both classifications are based on non-calcified nodules, with EUPS excluding calcified nodules from further evaluation and Lung-RADS describing them as category I nodules with benign characteristics and a less than 1% likelihood of malignancy requiring no additional monitoring.

Full table
The results of the randomised NLST study showed the possibility of raising the cut-off point for the size of nodule with a low probability of cancer (24). Similar observations can be made based on the results of the first two rounds of the NELSON trial (25). Therefore, for solid nodules detected at baseline LDCT scans, these significant volume thresholds are presented in Table 4.

Full table
Nodules of 100–300 mm3 volumes, for which the volume doubling time (VDT) is longer than 600 days, have not an increased risk of malignancy. Nodules with a VDT of 400–600 days having an intermediate cancer risk of about 4% and a second follow-up LDCT scan should be conducted within 3 months, as an initial management. The probability of developing cancer within 2 years in a nodule whose VDT is shorter than 400 days also depends on its volume, but ranges from 3–20% (25). Nodules smaller than 30 mm3 are not reported.
In indeterminate nodules detected at baseline studies, cancer eventually develops in 2–3% of lesions, while in the new incident nodules cancer is present three times more often (22). The Newly-discovered solid nodules can be divided into three groups of different risk using the size-based management approach (Table 5).

Full table
Nodules ≥200 mm3 have a high risk of cancer and require an interdisciplinary evaluation and additional diagnostics. Half of all detected low-risk and intermediate-risk new solid nodules display a benign character in the next LDCT study. In 7.0% of participants with non-resolving low-risk and intermediate-risk new solid nodules, the final diagnosis is lung cancer. Newly diagnosed nodules with an above-average risk of malignancy (i.e., a volume between 30 and 200 mm3) are subjected to another LDCT assessment with a volume measurement and VDT after 3 months. A new solid nodule with VDT ≤600 days, or an increase in volume to at least 200 mm3 represents a high probability of lung cancer and requires immediate referral to the interdisciplinary team. A test comprised of ≤ 600 days VDT cut-off, together with the predefined ≥200 mm3 volume cut-off for new incident nodules, reached sensitivity and negative predictive value close to 100% in terms of identifying lung cancer (26). If a nodule of volume greater than 30 mm3 is missed in the initial examination, it is recommended to calculate the time to double the volume to obtain further information on the risk and proceed according to the presented algorithm. The EUPS management recommendations of solid nodules are based on the existence of a proportional relationship between the initial volume, growth rate and risk of malignancy of these nodules (22).
For nodules incidentally detected in various clinical situations, guidelines provided by the British Thoracic Society (BTS) should be used (27). Similarly, the application of further guidelines from this association is used with sub-solid nodules in two different clinical situations, for both screened and clinically-detected nodules (28).
A solitary pure ground glass opacity (GGO) up to 5 mm does not require any follow-up CT study. If the patient has a high risk of developing lung cancer, annual LDCT is performed as part of the screening. To avoid misdiagnosing a solid nodule, it should be determined whether the lesion is true GGO by using contiguous thin CT sections. A solitary GGO larger than 5 mm requires an LDCT follow-up examination within 3 months, to establish the stable nature of the lesion and then annual CT testing for a minimum of 3 years is required if no solid fraction is observed in the follow-up. There is a predisposition towards growth when the size of the nodule is larger than 10 mm and where there is a history of lung cancer.
Sub-solid GGO, with a solid component greater than 5 mm, should be considered malignant until proven to be benign in character. Increase in size and other changes should be observed in the next CT scan performed within 3 months. Numerous GGO’s, with a size of 5 mm or less, should be observed with subsequent LDCT examinations carried out after 2 and 4 years. In cases where one of the GGNs is greater than 5 mm, a further LDCT examination is recommended within 3 months, followed by annual surveillance for at least 3 years. In multiple sub-solid nodules, among which dominant changes can be identified, the guidelines for the largest change should be followed. A control LDCT examination after 3 months is recommended, and in the case of solid fraction greater than 5 mm, aggressive diagnostic or therapeutic procedures should be applied.
Over the last four years, since LDCT lung cancer screening was introduced in the USA, the rate of recognition of GGOs has been improving. The presence of GGO lesions can suggest a diagnosis of premalignant, minimally invasive adenocarcinoma in the majority of cases, benign lesions in 20% of cases, and invasive adenocarcinomas in the rest. It is believed that there is a risk of multi-step progression in patients with long-term GGOs, which have the potential to transform from atypical adenomatous hyperplasia to adenocarcinoma in situ and finally to invasive adenocarcinoma (29). According to Henschke et al., all cancers manifest as part-solid nodules at repeat screening studies, all have begun as GGOs (30).
The management of non-solid lung nodules using different algorithms is based on both qualitative and quantitative methods, thus leaving more room for errors. Fortunately, in the case of non-solid nodules, due to the less malignant character of the lesions, there is more time for observation and treatment implementation.
Nodule management, invasive diagnostic procedures and treatment of screening detected lung cancer
Precise diagnostic algorithm that is applied for management of screen-detected nodules, majority of which are not malignant, should be highly sensitive not to overlook early stage lung cancer and highly specific to limit the false positive rate and in this way omit unnecessary invasive diagnostic procedures and surgery. Accuracy could be improved by positron emission tomography (PET) incorporation to the risk model in the selected cases (31). Implementation of the invasive diagnostic procedure should be maximally effective in a screening program, i.e., the number of unnecessary invasive procedures should be negligible. There are varieties of factors that influence the number of invasive diagnostic procedures in lung cancer screening participants. Nodule management protocol, quality of CT machines, slice thickness and experience of the screening team determines the prevalence of detected nodules and influence directly and indirectly the frequency of undertaken invasive procedures. The most important factors however in reducing harmful diagnostics are strict adherence to the radiological and nodule management protocols combined with the screening team’s experience.
Frequency of the detected noncalcified nodules in the LDCT screening studies varies between 21% and 56% in the baseline round as a result of different inclusion criteria and the size detection limit (32-34). The number of solid new noncalcified nodules in the incidence rounds varied between 3% and 11% (35,36). In each of stated situations, the proceedings should be quite different. New detected nodules requires more attention due to higher incidence of lung cancer ranging between 4–6% comparing to a mean of 2% in the majority of the reported baseline rounds. Ground glass nodules are another category of lung lesions that needs different assessment protocol and diagnostic policy that is clearly defined both in ESTS guidelines and in polish consensus statement (6,7).
The number of participants that are sent to invasive diagnostics depende on the nodule management and on sticking to the management protocol. The experience of multidisciplinary team members with a focus on radiologist and surgeon is of utmost importance. In the program performed in Gdańsk between 2009 and 2011, 3.2% of all screenings were sent to diagnostic workup and in 14.5% of them lung cancer was diagnosed. In the second program performed between 2016 and 2018, 3.5% were sent to diagnostic work up and in 52.7% lung cancer was diagnosed (13). This shows the importance of experience in the implementation of the screening program.
Reducing the number of unnecessary operations in benign tumors and performing mini-invasive and lung sparing procedures are the primary tasks of surgeons in the screening program. Therefore, thoracic surgeons participating in the screening program should meet a number of criteria precisely defined in the ESTS guidelines, among which the training in minimally invasive techniques and knowledge of oncological guidelines play a key role (6). It is possible only in highly specialized departments of thoracic surgery. Surgeons that participating in the multidisciplinary teams should be highly specialized in VATS operations that has proved to be at least as effective in the oncological aspect simultaneously being significantly less harmful for the patient (37). The indications to perform sublobar resections in lung cancer patients are very limited currently in the light of available evidence but the results of 2 randomized controlled trials: Japan Clinical Oncology Group (JCOG 0802) and Cancer and Leukemia Group B (CALGB 140503) should be available before 2023 (38). In this particular situation where a handful of very early stage lung cancers are diagnosed and the gold standard lung cancer treatment is still lobectomy surgeon’s decision is crucial. In the majority of publications analyzing long term survival of lung cancer patients operated with tumors that are less than 1–2 cm the outcomes of patients operated with limited resection are at least as good as after lobar resection. Another approach should be applied to GGO lesions which are a form of pre-invasive lung cancer until they are transformed into part solid nodules. Sublobar resection with intraoperative pathology assessment in these lesions should be applied in the majority of cases. In each lung cancer screening patient thoracic surgeon should tailor the treatment strategy considering the individual patient prognostic factors including age, comorbidities, performance status and life expectancy.
Lung cancer screening participant should be provided with optimal assistance and care of the multidisciplinary team. If a lung cancer is detected, a team consisting of an oncologist, radiotherapist and surgeon should provide him with a whole range of possible therapeutic solutions. In the light of satisfactory results in the treatment of early lung cancer with stereotactic body radiation therapy, this option should also be carefully discussed particularly with compromised patients. There are possible applications of other therapeutic options in the future as radiofrequency ablation, especially in GGO lesions, but their usefulness must be confirmed in a prospective manner.
Smoking cessation program
Obviously, it can be taken for granted that primary prevention must not be separated from the secondary one. Therefore any lung cancer screening program should be linked with smoking cessation.
For the participant, lung cancer screening is often the first significant intervention that brings attention to smoking-related health harms. Participants of the screening program have to absorb a lot of information, perception of which, and psychological effects on behavior and decisions have not been fully studied. The risk that lung cancer screening may be regarded as a surrogate to smoking cessation to reduce mortality, has been recognized and confirmed by many authors (39).
The costs of adding a cessation intervention to an LDCT lung cancer screening program increase the total cost by a few to over a dozen percent. However, the cost-effectiveness as measured by quality-adjusted life years increases 1.7- to 5.4-fold (40).
We propose the particular procedure for smoking cessation intervention as part of the screening program (7). First, each screening has to be assigned to one of the specific intervention group on the basis of self-declaration: active smoker, former smoker-abstinent for less than 12 months, and former smoker-abstinent for more than 12 months.
Active smokers undergo a standardized interview to assess smoking intensity and the severity of dependence (Fagerström). It is complemented by a test of motivation for smoking cessation (Schneider), measurement of exhaled carbon monoxide (Smokerlyzer) or salivary cotinine and an assessment of depression (Beck’s scale).
On the basis of the above evaluations, patients are adequately supported in the form of consultation, psychotherapy sessions and education. Pharmacological support includes nicotine replacement therapy as the first-choice treatment, and cytisine, varenicline and bupropion if previous treatment failed. Patients with a positive evaluation of depression are advised to register at an outpatient psychiatric facility.
Consequently, patients declaring abstinence from smoking for less than 12 months undergo Smokerlyzer testing (or cotinine testing) and once nonsmoking status is confirmed, they take a test measuring their motivation to continue not to smoke and undergo a short intervention reinforcing their decision to quit smoking.
Patients declaring abstinence from smoking for more than 12 months receive a consultation to reinforce their motivation to maintain the non-smoking status.
All the individuals being considered for the screening program are inquired about the exposure to passive smoke in the workplace or at home. If such exposure is identified, an invitation is extended via the patient to the patient’s relatives and co-workers to participate in the cessation intervention.
Data collection, storage and sharing
Big data analysis has successfully entered many fields of clinical medicine. This, in part, has been fueled by the growing exploitation of artificial intelligence. Especially its fundamental concept, i.e., machine learning turned out to be helpful in pattern recognition and thus capable of disclosing the intricate correlations in medical image and alphanumeric data sets. The resultant outcome leads, for instance, to semi-automation in image analysis, thus decreasing the staggering burden of SPN identification and assessment.
As there are multiple options and algorithms being tested in machine learning, it is crucial to make an accumulated data base accessible to the researchers’ community under the established rules. Networking and exchanging data globally will bring lung cancer screening community closer to discovery of the high throughput tools enabling to handle the exponentially increasing amount of accrued data.
As a part of preparation process to launching the early lung cancer screening program in Poland we have prepared our own solution. The Screening Registry is integrated, comprehensive, turnkey solution developed to monitor and report on the diagnostic and therapeutic process and its results under Polish Early Detection of Lung Cancer Program (EDLC Program) employing LDCT. The goal of the Screening Registry is to store and process medical records of all patients screened for lung cancer in all Centers for Screening of Lung Cancer (CSLC). The EDLC Program assumes that CSLCs will be reporting data on all screening studies including their key findings to a single Screening Registry in order to conduct financial settlement of the performer procedures and to monitor quality and efficiency of the Program. Collected data will be also made available for scientific research purposes. It is expected that obtained data will be helpful in detailed planning of the lung cancer prevention and treatment country-wide. The Screening Registry is architected as a secure, cloud-based, turnkey solution that provides an access to scalable computing and storage infrastructure and to integrated products and services that support functional assumptions of EDLC Program. It is composed of separate application modules dedicated to different users and organizations, depending on their role in the Screening Program and their technological capability. The system also contains a separate module for patients that have been admitted to the Screening Program. The Screening Registry can be used as a standalone and complete, fit for the purpose solution thanks to its data entry facilities, providing an access for multidisciplinary team members and patient history tracking. The Registry has built-in tools for structured data entry, editing and validation (Master Patient Index, RadLex, Structured Reports, Voice Recording and Transcription, Lung RADS Support, etc.). Regardless of the local availability of DICOM & HL7 interfaces, medical personnel of Screening Centers have access to the Screening Register via public internet. The connection to the Registry is SSL-encrypted and requires only a web browser and a valid user account.
User access to the Registry is managed by the central SSO-module. Devices connected to registry are encrypted and managed by network authorization and management module.
Polish National Demonstration Program
LDCT lung cancer screening is a new challenge in medicine, which entered in this decade into the phase of widespread application. The experience in this completely new field has been collected in the limited number of centers worldwide. This experience requires reviewing hundreds of LDCT studies by radiologists, gaining experience in diagnosing small lung tumors often located in parts of the lung very inconvenient for biopsy and finally making appropriate therapeutic decisions or the decisions of further observation of the suspected nodule.
Therefore, in the phase of introducing screening program, the first option seems to be to introduce it gradually, starting from one center and then attaching subsequent units, after appropriate training. Another solution that guarantees a high quality program at the beginning of its implementation is the use of a centralized image reading in one or few experienced radiological centers where other radiologists, who are interested in taking part in screening, are simultaneously trained.
The cooperating team of all specialists involved in this process is essential for making the right decisions. Therefore, the sine qua non condition is a multidisciplinary team operating in such a center that should include thoracic radiologists, pathologists, pulmonologists, thoracic surgeons, medical oncologists, radiation oncologists and nurses who are experienced in lung cancer management and are trained in the process of screening.
Anti-tobacco intervention is another issue that should be a component of any screening program. Its effectiveness depends on the team’s involvement in this process. The optimal solution seems to be a model in which the screening participant will receive appropriate intervention at every stage of contact with the personnel supporting such a study. In addition to information about the harmfulness of smoking, which should be meticulously prepared, he should also receive a leaflet or information book about the harmfulness of smoking, as well as methods of effectively quitting smoking. This is the basic version that must be used in each case. The optimal solution is complex intervention, which additionally involves setting an appointment and directing all interested parties to the tobacco addiction treatment in appropriate outpatient clinic. The solution to this problem is complex and requires a lot of organizational efforts. In the Polish program, complex intervention will be available at most in 2/16 places where a screening program will be held due to the lack of such a anti-tobacco clinics in Poland.
Radiological examinations and their interpretation require the experience of a radiologist based on hundreds of reviewed studies. Such experience is the result of several years of work supported by participation in discussions on diagnostics, treatment and follow up the effects of this activity. In the Polish program, in which the examinations will be carried out in 16 centers selected on a tender basis, only 4 centers have previous experience in conducting LDCT lung cancer screening. That is why we offered central reading of radiological images. It is difficult to find a radiologist or a team of radiologists who is entirely dedicated only to reading LDCT from screening. Therefore central reading in one department is impossible so far in Polish conditions hence the proposal to designate few such centers. For radiologists from the remaining centers, teaching files and training in the aforementioned 2–4 radiology departments will be prepared.
An extremely important element of introducing screening program in a centralized system as introduced in Poland, which differs from the one proposed in the USA, is data system archiving radiological images. This has a twofold significance. On the one hand, it is an important element allowing to control the quality of operations of individual centers, and on the other hand allows to create a base for future research. In addition, the archiving program should be developed with the operation of a network program allowing the input of epidemiological, diagnostic and therapeutic data from different places and the ability to read these data by all specialists participating in the screening from the family doctor to the surgeon. This possibility is created by programs specially designed for screening. To our knowledge, there are two such programs designed in US and Holland. An alternative is to use any medical program operating in a given country adapted to the screening program. Undoubtedly, the program dedicated to screening has superiority but its development is cost- and time-consuming. Implementation of a program already developed beyond the borders of a given country is associated with many different aspects related to the costs and regulations regarding the protection of personal data (GDPR). The storage of radiological images is possible in the cloud and such a service is available in all countries prepared by specialized IT companies in radiology. In Poland, we will use the services of an external company that provide such a service.
In the ESTS recommendations and EU position statement o lung cancer screening, a lot of attention is paid to the quality assessment and accreditation of research centers as well as specialists involved in screening (6,7,22). In the Polish program, the initial quality assessment will be carried out at the stage of selection of centers for conducting the program, which will be carried out on the basis of a competition in which CT technical equipment requirements, presence of multidisciplinary teams in these centers, possibility of antitobacco interventions as well as the need to sign a contract with at least 40 centers of family medicine will be required. Further quality control will be developed during the program and the accreditation rules will be derived from it.
The biggest target problem of screening lung cancer using LDCT is recruitment for this study. In the USA, in the first year of the program implementation, only 1.9% of those eligible for such intervention were tested (41). We have similar observations in Gdansk. After great interest in the first program implemented in 2009–2011, the interest was huge, exceeding the recruitment possibilities. In the second program implemented in the same province in 2016–2018, the problems with recruitment were significant because the participants of the previous program were no longer eligible for recruitment. Therefore, we pay great attention to participation of the family doctors in the program, which is crucial for sending patients at high risk of developing lung cancer for screening. In England, the pilot screening program that has been announced is planned to use CT-buses—we will be looking in Poland at the results of this undertaking with great interest.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- Pyenson BS, Henschke CI, Yankelevitz DF, et al. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits 2014;7:272-82. [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- Kauczor HU, Bonomo L, Gaga M, et al. European Society of Radiology (ESR), European Respiratory Society (ERS), European Society of Radiology (ESR), European Respiratory Society (ERS). ESR/ERS white paper on lung cancer screening. Eur Radiol 2015;25:2519-31. [Crossref] [PubMed]
- Pedersen JH, Rzyman W, Veronesi G, et al. Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. Eur J Cardiothorac Surg 2017;51:411-20. [PubMed]
- Rzyman W, Didkowska J, Dziedzic R, et al. Consensus statement on a screening programme for the detection of early lung cancer in Poland. Adv Respir Med 2018;86:53-74. [Crossref] [PubMed]
- Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485-91. [Crossref] [PubMed]
- Tammemägi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging 2015;30:88-100. [Crossref] [PubMed]
- Plumb AA, Halligan S, Nickerson C, et al. Use of CT colonography in the English bowel cancer screening programme. Gut 2014;63:964-73. [Crossref] [PubMed]
- Miglioretti DL, Gard CC, Carney PA, et al. When radiologists perform best:the learning curve in screening mammogram interpretation. Radiology 2009;253:632-40. [Crossref] [PubMed]
- Esserman L, Cowley H, Eberle C, et al. Improving the accuracy of mammography: volume and outcome relationships. J Natl Cancer Inst 2002;94:369-75. [Crossref] [PubMed]
- Ostrowski M, Marjanski T, Dziedzic R, et al. Ten years of experience in lung cancer screening in Gdańsk, Poland: a comparative study on evaluation and surgical treatment of 14 200 participants of 2 lung cancer screening programmes. Interact Cardiovasc Thorac Surg 2019. Epub ahead of print. [Crossref] [PubMed]
- Yoo RE, Goo JM, Hwang EJ, et al. Retrospective assessment of interobserver agreement and accuracy in classifications and measurements in subsolid nodules with solid components less than 8mm: which window setting is better? Eur Radiol 2017;27:1369-76. [Crossref] [PubMed]
- Singh S, Pinsky P, Fineberg NS, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology 2011;259:263-70. [Crossref] [PubMed]
- Rampinelli C, Origgi D, Bellomi M. Low-dose CT: technique, reading methods and image interpretation. Cancer Imaging 2013;12:548. [Crossref] [PubMed]
- Zhao YR, Heuvelmans MA, Dorrius MD, et al. Features of resolving and nonresolving indeterminate pulmonary nodules at follow-up CT: the NELSON study. Radiology 2014;270:872-9. [Crossref] [PubMed]
- Berrington de González A, Kim KP, et al. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen 2008;15:153-8. [Crossref] [PubMed]
- Jin S, Zhang B, Zhang L, et al. Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg 2018;8:480-90. [Crossref] [PubMed]
- Fujita M, Higaki T, Awaya Y, et al. Lung cancer screening with ultra-low dose CT using full iterative reconstruction. Jpn J Radiol 2017;35:179-89. [Crossref] [PubMed]
- Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347-56. [Crossref] [PubMed]
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- McKee BJ, Regis SM, Mckee AB, et al. Performance of ACR Lung-RADS in a Clinical CT Lung Screening Program. J Am Coll Radiol 2015;12:273-6. [Crossref] [PubMed]
- Church TR, Black WC, Aberle DR, et al. NationalLungScreeningTrial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, ten Haaf K, et al. Persisting new nodules in incidence rounds of the NELSON CT lung cancer screening study. Thorax 2019;74:247-53. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Bettio D, Venci A, Achille V, et al. Lung cancer in which the hypothesis of multi-step progression is confirmed by array-CGH results: a case report. Exp Ther Med 2016;11:98-100. [Crossref] [PubMed]
- Henschke CI, Yip R, Smith JP, et al. International Early Lung Cancer Action Program Investigators. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 2016;207:1176-84. [Crossref] [PubMed]
- Al-Ameri A, Malhotra P, Thygesen H, et al. Risk of malignancy in pulmonary nodules: a validation study of four prediction models. Lung Cancer 2015;89:27-30. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Rzyman W, Dziedzic R, Jelitto-Górska M, et al. Results of an open-access lung cancer screening program with low-dose computed tomography: the Gdańsk experience. Pol Arch Med Wewn 2015;125:232-9. [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl Lung Cancer Res 2017;6:42-51. [Crossref] [PubMed]
- Baldwin DR, Devaraj A. Lung cancer risk in new pulmonary nodules: implications for CT screening and nodule management. Lancet Oncol 2016;17:849-50. [Crossref] [PubMed]
- Dziedzic R, Marjański T, Binczyk F, et al. Favourable outcomes in early-stage non small cell lung cancer patients operated by VATS - a propensity score-matched analysis. Eur J Cardiothorac Surg 2018;54:547-53. [PubMed]
- Nakamura K, Sajii H, Nakajima R, et al. A phase III randomised trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and Perceptions About Smoking Cessation in the Context of Lung Cancer Screening. JAMA Intern Med 2015;175:1530-7. [Crossref] [PubMed]
- Goffin JR, Flanagan WM, Miller AB, et al. Biennial lung cancer screening in Canada with smoking cessation-outcomes and cost-effectiveness. Lung Cancer 2016;101:98-103. [Crossref] [PubMed]
- Pham D, Bhrandari S, Oechsil M. Lung cancer screening rates: Data from the lung cancer screening registry. ASCO Meeting Library presented 2018.06.01.


