Implementing smoking cessation within cancer treatment centres and potential economic impacts
Introduction
The evidence that smoking cessation improves cancer treatment outcomes is irrefutable. The 2014 US Surgeon General’s report summarized that evidence and concluded that continued smoking after a diagnosis of cancer can result in an increase in all-cause and cancer-specific mortality, greater toxicity from therapeutic interventions and an increased incidence of recurrence and second malignancies (1). The report further states that the risk of dying could be lowered by 30% to 40% by quitting smoking at the time of cancer diagnosis. Since the Surgeon General’s report, additional evidence has accumulated on the adverse outcomes of failure to quit smoking in cancer patients for a variety of different tumour types, including some that are not smoking related (2-5). The accumulation of evidence provides a strong rationale for making smoking cessation a standard component of treatment within all cancer centres and that all cancer patients be screened for their smoking status, advised of the health benefits of cessation and provided with help in quitting. Support should also be provided to patients who have recently quit smoking, due to the high probability of relapse (6).
Further impetus for efforts to integrate smoking cessation initiatives into cancer treatment facilities has come from the National Cancer Institute (NCI) Moonshot Program. Funding has been provided through the Cancer Centre Cessation Initiative to more than 40 NCI-designated cancer centres during 2017 and 2018, to implement programs that help cancer patients to stop smoking (7). The NCI recognized that despite the advances that have been made in cancer treatment, the failure to assist patients to stop smoking at the time of diagnosis is a core gap in cancer care.
Although virtually all oncologists ask patients about their smoking behaviours, relatively few feel comfortable assisting their patients in stopping smoking. A survey of lung oncologists through the International Association for the Study of Lung Cancer showed that 81% advise their patients to quit, but only 39% actively provide cessation assistance, and fewer yet address tobacco use at follow-up (8). The principal barriers to physicians promoting smoking cessation are pessimism regarding their ability to help patients stop using tobacco (58%), and concerns about patient resistance to receiving advice about smoking cessation treatment (67%). Only a third of thoracic oncologists felt that they were adequately trained to provide cessation interventions (counselling and pharmacotherapy) (8).
For smoking cessation to become a standard part of treatment for cancer patients, it will be necessary to educate cancer patients and oncology healthcare providers about the health benefits of smoking cessation, and to put in place resources that do not unduly burden already very busy oncology practices.
Methods and results
Introducing smoking cessation to Ontario’s regional cancer centres
In 2011, Cancer Care Ontario (CCO), the government agency responsible for the quality of cancer care in the province of Ontario, Canada, held a clinical planning day at which the potential benefits of smoking cessation, documented in two seminal papers, were presented (9,10). The data in these two papers provided convincing evidence of a survival benefit of smoking cessation in patients with lung and head neck cancer. Based on this information, CCO established a Steering Committee tasked with developing an implementation framework for a smoking cessation initiative (the “initiative”). The committee, chaired by one of the authors (WK Evans), included experts in the field of smoking cessation from the University of Ottawa Heart Institute (a Canadian leader in smoking cessation responsible for the “Ottawa Model for Smoking Cessation”), the Centre for Addiction and Mental Health in Toronto, the Registered Nurses’ Association of Ontario and the Canadian Cancer Society. As well, researchers, oncologists and healthcare administrators interested in advancing the initiative were invited to participate. Over a 6-month period of regular meetings, the committee reviewed the medical literature on the health benefits of smoking cessation, produced a vision statement and developed a framework for the initiative with a set of implementation recommendations. The vision statement—“By systematically offering a smoking cessation intervention to every ambulatory cancer patient, the Regional Cancer Program Smoking Cessation initiative will help to ensure that cancer patients in Ontario achieve the best possible health benefits from their cancer treatments”—was later endorsed by regional smoking cessation champions and the program’s Advisory Committee.
The challenge of realizing this vision was significant in Ontario, which has a population of 13.5 million and 14 health regions each with a regional cancer centre, as well as numerous satellite cancer treatment facilities varying in size and capacity. Based on 2011 to 2014 data from the Canadian Community Health Survey, on average, one in five current cancer patients (20.1%) reported daily or occasional smoking (11). This percentage was comparable to that of the general population, which reported a 19.3% daily or occasional smoking rate (12). Data collected by Cancer Care Ontario in 2018 showed that one in six current cancer patients (16.5%) reported current smoking or smoking in the past 6 months (13). The initiative targeted new ambulatory cancer patients. This included anyone registering at an Ontario cancer centre for the first time, or anyone registering more than 12 months after a previous registration date regardless of the type of malignancy. Smoking rates and the frequency of smoking-related malignancies vary across the province, with smoking rates being higher in the North and in health regions where there has historically been a greater concentration of manufacturing and heavy industry. To implement a smoking cessation initiative simultaneously in all cancer centres in Ontario required considerable effort, and there have been numerous learnings. This paper describes the steps taken by CCO to implement the smoking cessation initiative and provides information on the potential economic impacts. The learning process continues as individual cancer centres experiment with strategies to increase tobacco screening and cessation referral rates, and as research is undertaken to determine the impacts of the initiative.
Key steps in successful implementation
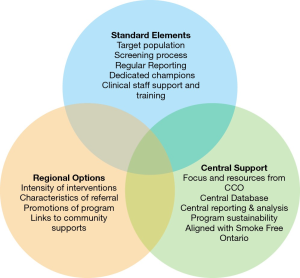
The first steps in establishing a smoking cessation initiative are to have the guidance of smoking cessation experts and a framework to guide those charged with implementing the initiative. The Steering Committee recommended a framework with three broad components, as shown in Figure 1: required standard program elements; suggested but optional regional initiatives; and central administrative support. The standard elements required by all cancer centres were that they target new ambulatory cancer patients, use a standardized tobacco screening question to identify current and recent smokers, designate a regional smoking cessation “champion”, train healthcare providers on how to empathetically interact with patients who smoke, and provide a referral for patients who accepted smoking cessation services. Regular submission to CCO of data on performance metrics was also required.
Given the diversity in size and demographics of the population served by the regional cancer centres, facilities were provided with flexibility regarding some aspects of the initiative. These included the intensity of the smoking cessation intervention offered, which could range from brief to intensive; the characteristics of the referral (whether patients were referred to smoking cessation services within the cancer centre or host hospital, or to an external smoking cessation service, such as a community-based program or the provincial quit line); which agencies to partner with in the local community; and how best to promote the initiative and mitigate risks at the regional level.
CCO established central administrative support, with a small dedicated team within the Prevention and Cancer Control portfolio, and a central database within Analytics and Informatics. The CCO team was supported and guided by an Advisory Committee of smoking cessation experts (which evolved from the Steering Committee), as program funding became available to implement the program in the regions.
No new funds were provided to the 14 regional cancer centres to begin this concerted provincial effort on smoking cessation. Instead, regions were asked to direct funds that had previously been used to support generic cancer prevention initiatives towards smoking cessation. Cancer Care Ontario was aware that the small amount of funds being used by each cancer centre was not enough to support a comprehensive smoking cessation program with an in-house quit coach. A business case was developed for the provincial government for additional financial support. After several yearly submissions, confirmation was received from government that smoking cessation would receive base funding support in all cancer centres. Positioning smoking cessation as a key component of quality cancer care leading to improved outcomes for patients was what eventually garnered the increase in financial support for the smoking cessation program.
The six recommendations that guided the initial implementation of the initiative were as follows:
- Screen all new ambulatory cancer patients for smoking status;
- Standardize the screening and referral questions and data collection;
- Monitor the effectiveness of the smoking cessation initiative;
- Develop and maintain an inventory of the available smoking cessation services within each health region;
- Establish external partnerships;
- Take steps to minimize the barriers to implementation and the risks to sustainability.
It was recommended that all new ambulatory cancer patients be assessed for smoking status by a nurse or physician, and that the 5 As (ask, advise, assess, assist, arrange) model of smoking cessation be used. However, this model was not prescribed, and some centres chose to implement tobacco screening by support staff at the time of registration in order to reach more patients. Performance metrics were defined, and centres were required to report on a minimum data set monthly, with the data being analyzed and reported quarterly. The actual screening and referral process were not standardized, and was implemented differently across the centres—some used electronic systems, while others used paper-based systems. However, standardization of screening and referral questions was largely accepted, and centres provided staff with lanyard cards and other tools with recommended scripting to prompt them to ask the appropriate questions. The standardized screening question in the original framework was “Have you used tobacco products, such as cigarettes, pipes, cigars, or chewing tobacco, in the last 6 months?” For comparability of data for reporting purposes, a “current or recent smoker” was defined as an individual who had used tobacco in the previous 6 months. In order to assess a patient’s willingness to quit and offer a referral, the standard question was “Are you interested in learning about what is available to help you avoid smoking/using tobacco in the future?” The centres were encouraged to develop an inventory of regional smoking cessation resources, and to keep that inventory current. However, the way that this was implemented was dependent on identified local factors. As Figure 1 shows, links to community supports were considered a component of the program that allowed for regions to choose approaches that were a best “fit” for their program but implementation of this component of the program was not monitored by CCO. Most regions had access to a variety of such services, including the Canadian Cancer Society’s Smokers’ Helpline (a phone, web and text-based service), trained pharmacists and family physicians, public health units, and hospital and community-based smoking cessation clinics. One regional cancer centre attempted to connect with every privately run pharmacy in their region. As a result of this exercise, a “roster” of pharmacists willing to provide smoking cessation counselling to cancer patients was established. The regional cancer programs were encouraged to partner with these other service providers, as the centres themselves often did not have the funding to resource their own smoking cessation counsellors or to provide smoking cessation medications.
Important key learnings from CCO’s experience with implementation included the need for senior-level commitment and infrastructure support, the value of performance metrics to drive change, the need to educate and re-educate healthcare providers on the rationale for the initiative, and the need to continue to evolve the smoking cessation initiative. Each of these learnings is discussed below.
Senior-level commitment and infrastructure support
Given the multiplicity of demands and the competition for resources within the provincial cancer system, it was extremely important that the senior leadership of CCO and the regional cancer programs all demonstrated a commitment to the initiative. The Provincial Leadership Council (CCO executives and regional cancer program leaders) endorsed the establishment of a secretariat and committed to the framework recommendations developed by the Steering Committee.
Commitment to the framework was further cemented by including specific language and deliverables in funding and accountability agreements that were signed by executives at Cancer Care Ontario and each regional cancer centre. Expectations and deliverables outlined in these agreements included key aspects of the program such as, the designation of a regional smoking cessation champion, connecting with regional clinical leads in surgery, systemic and radiation therapy and the regular reporting of patient-level smoking cessation data.
Provincial infrastructure support consisted of a smoking cessation secretariat at the provincial office of CCO. The secretariat’s manager and staff had expertise in health promotion and epidemiology. They organized regular meetings of the Advisory Committee and the smoking cessation champions to communicate the goals of the initiative, to develop educational materials for cancer patients, to establish and maintain a smoking cessation dataset within CCO Informatics, and to develop, capture, analyze and report on the smoking cessation performance metrics (described below). The secretariat also engaged with CCO’s Informatics department and the region’s data leads to address data submission and/or data quality issues.
The regional champions were a key component of regional infrastructure support for the program. Champions could be of any professional discipline and were selected for their enthusiasm to lead the initiative, their knowledge and training in smoking cessation, and excellent communication skills. Their responsibilities included successful engagement of staff at various levels (medical, nursing and allied health professionals, clerical and information technology), and leading implementation of the framework in their region. Regional champions worked diligently to assess and report on the current state of smoking cessation activity and collaborated with Cancer Care Ontario provincial office staff as required. Monthly phone or web-based meetings of all regional champions were integral in achieving the provincial program goals. Meetings allowed for knowledge exchange on effective practices, the development of patient resources, and the best techniques to engage healthcare providers. Annual in-person meetings with the champions have been held to discuss best practices and address common issues and concerns using a workshop or problem-solving approach. Best practices were identified by examining the intervention processes of the highest-performing centres on each of the indicators. For example, the two cancer centers with the highest tobacco use screening rates both used clerical staff to screen new patients during a centralized registration process. The highest levels of acceptance of referrals occurred at centres with an onsite dedicated smoking cessation counsellor, suggesting that cancer patients may prefer to attend a cessation service offered within the cancer center itself, rather than one requiring travel to an external community service.
The value of performance metrics
The Steering Committee initially recommended five key performance metrics: (I) the proportion of ambulatory cancer patients screened for their smoking status; (II) the proportion of those screened who were current or recent smokers; (III) the proportion of current or recent smokers who were advised to quit smoking; (IV) the proportion of those advised to quit who were recommended a referral to smoking cessation services; and (V) the proportion of those offered a referral who accepted the referral. If a referral was accepted, the type of referral (internal to the cancer centre or host hospital, or external to a provincial or community-based service) was also captured. After 2 years of monitoring these metrics, the Advisory Committee recommended revisions to increase the focus on program outcomes. A focus was placed on the proportion of all current and recent smokers who were advised to quit, recommended a referral, and accepted a referral for cessation support. Two of the metrics (tobacco screening and accepted a referral) are reviewed quarterly by senior CCO executives with the regional cancer program leaders in order to drive change. Targets have been set and the performance on these two indicators is included in the overall ranking of Ontario’s 14 cancer centres four times a year. This approach encourages friendly competition as centres attempt to be amongst the top performers.
In addition to the central monitoring of the five performance metrics noted above, several of the 14 cancer centres track regional program outcomes. For example, cancer centres that have on-site cessation counselling are able to follow-up at regular time intervals with patients, monitor patients’ quit attempts and calculate quit rates. While capturing patient outcomes is beyond the scope of CCO’s provincial program, efforts are currently underway to build this into the initiative.
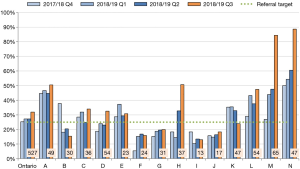
Current data on the proportion of ambulatory cancer patients screened for smoking status demonstrates that most centres have achieved the target of 75%; however, despite high volumes screened, the two largest centres are struggling to reach this target. The second key performance metric at the regional quarterly review is the proportion of smokers who accepted a referral to cessation support. As shown in Figure 2, many centres are struggling to achieve the current target of 25%. Poor performance on this indicator led the initiative to adopt an “opt-out” approach to referrals, in which all smokers are automatically referred to a smoking cessation service unless they specifically refuse. This is a recent strategy and the early adopters are demonstrating increased referral acceptance rates.
The need to educate healthcare professionals
The benefits of smoking cessation in cancer patients have been well documented, in publications such as the American Association for Cancer Research policy statement (14) and the Surgeon General’s 2014 report (1), but this information was not well known by most healthcare providers working in Ontario’s cancer system and was not influencing their practice. The regional champions led in-service trainings, particularly with nurses and other allied health professionals who were often tasked with completing the smoking cessation intervention with patients. To complement this, a variety of initiatives were undertaken by CCO to educate healthcare providers, including lectures in the regional centres, a blog post, a publication on the implementation of the initiative (15), presentations at national and international meetings and the creation of videos, posters and patient materials. One cancer centre staged a debate between their head of medical oncology and the chair of CCO’s smoking cessation Advisory Committee. This was very successful in increasing the attendance of the centre’s oncologists, something which had not been achieved in other centres when the topic of grand rounds was advertised as “smoking cessation”.
Due to frequent turnover of personnel in cancer centres, there is a need for continual re-education. Training modules have been created and education on smoking cessation is mandated for new hires in many of the centres and integrated into new staff orientation.
Despite these efforts, it has still been difficult to motivate healthcare providers, particularly oncologists, as many continue to express concerns about workload and their ability to take on additional tasks. Presentations have emphasized that the intervention can and should be brief, and follow a script that informs patients that one of the most important things they can do to get the best results from their cancer treatment is to quit smoking. Oncologists have been encouraged to promptly connect the patient to an appropriate smoking cessation resource within the cancer centre, hospital or community setting. It must be acknowledged that oncologists, in particular, play a critical role in motivating their patients when they speak directly to them about the importance of smoking cessation. When they do not, it undermines the efforts of all other healthcare providers who are working to help the patient to quit smoking.
The need to continuously evolve the program
As time has passed, it has become evident that the program needs to continuously evolve both in its structure and scope. Early on, it was apparent that capturing five performance metrics (defined above) was work intensive and that some metrics were of greater importance in demonstrating how well centres were implementing the initiative. The key metrics driving performance were the proportion of new patients screened for smoking status, and the proportion of smokers referred to cessation services. However, what is even more critical than knowing whether a smoker has accepted a referral is information on whether they have quit or made a quit attempt. Over the next year, the program will focus on gathering more information on quit attempts.
Following a process review 1 year after the program launched, it was acknowledged that the use of the 5 As was a barrier to full implementation in busy cancer centres. Guidance from the UK National Health Service recognized that a 3 As (ask, advise, act) approach is as effective as the more time-consuming 5 As approach (16). In 2017, the Framework was revised to align with the 3As approach. The standard tobacco screening question was updated to a simpler version, “Have you used any form of tobacco in the last 6 months?” It was also observed that staff often felt reluctant to ask patients about their willingness to quit smoking or accept a referral. Consequently, an “opt-out” approach to referrals was introduced, where smokers are automatically referred for cessation support unless they refuse. The referral script was also updated to be a statement, rather than a question: “I’m going to refer you to.. (insert available smoking cessation service).” This has improved the accepted a referral rate and it is anticipated that it will increase further as staff become more comfortable with this approach.
It is also important that the scope of the program evolve over time. Currently, the program targets new ambulatory cancer patients, who typically receive only a single smoking cessation intervention within 28 days of their first consultation at a cancer centre. Within this timeframe, new patients are confronted with a great deal of information about their diagnosis and treatment and for some it may be an inopportune time for them to consider smoking cessation. Ideally, oncologists and other healthcare providers should ask patients about their smoking status at each cancer centre visit and continue to encourage patients to accept a referral to help them quit smoking. It is expected that as the culture within cancer centres evolves, ongoing smoking cessation interventions will become the norm rather than the exception. In fact, some regional cancer centres have already made modifications to their program to do this. In addition, patients who continue to smoke and those who received treatment prior to the start of the initiative should also be recruited into the program so that they too can accrue both short and long-term health benefits.
The smoking cessation program could also consider expanding its target population to include individuals attending cancer screening and diagnostic programs. CCO is responsible for provincial breast, colorectal and cervical screening programs and is currently conducting a pilot of low-dose CT screening for lung cancer. While smoking cessation is incorporated into the lung cancer screening pilot, it has not yet been integrated into the other screening programs where the potential benefits could be even greater by preventing cancers and other smoking-related illnesses.
Discussion
As healthcare costs escalate in publicly funded healthcare systems, it has become increasingly important to prove that any new cancer control intervention is not only clinically effective, but also cost-effective or cost saving. It has been well established that smoking cessation is a cost-effective intervention in the general population (17). There is also abundant evidence that hospital-based interventions are highly effective at helping patients quit smoking, particularly when pharmacotherapy is provided in combination with counselling and post-discharge support. One example of this is the Ottawa Model for Smoking Cessation (OMSC), which has been adopted in over 120 hospitals across Canada. The OMSC involves identifying and documenting the smoking status of all patients, providing brief counselling and in-hospital pharmacotherapy to smokers, and offering follow-up support after hospitalization. This model has been shown to improve the long-term cessation rate by an absolute 11% (from 18% to 29%; odds ratio, 1.71; P=0.02) among general hospital patients (18). Furthermore, in a two-group effectiveness study, it has been demonstrated that those receiving the OMSC experience significantly lower rates of all-cause readmissions, smoking-related readmissions and all-cause emergency room visits. A cost-effectiveness analysis based on a decision-analytic model to assess smokers hospitalized in Ontario showed that delivery of the OMSC could be considered cost-effective with a 1-year cost per quality-gained of $1,386 and a lifetime cost per quality-gained of $68 (19). In the first year of an OMSC program, it was calculated that provision of the program to 15,326 smokers would generate 4,689 quitters and would prevent 116 rehospitalizations, 923 hospital days and 119 deaths. The OMSC, which has similar characteristics to the CCO smoking cessation initiative (except for the post-discharge follow-up), appears to be cost-effective from the hospital perspective; one would surmise that smoking cessation within the cancer centre context would be similarly cost-effective. However, there is a limited amount of data on the cost and cost-effectiveness of smoking cessation in the cancer context.
To address this deficiency, a team led by one of the authors (W Isaranuwatchai) has worked to generate economic evidence in four areas: (I) a systematic literature review of economic evaluations of smoking cessation programs in the oncology setting to confirm that there is a knowledge gap; (II) a real-world analysis of the impact of smoking on health care costs among cancer patients to identify the potential economic impact on the health system (20); (III) a cost-effectiveness analysis of smoking cessation in Ontario’s regional cancer programs (paper in preparation); and (IV) an estimate of the cost-effectiveness of implementing various smoking cessation strategies among cancer patients.
The systematic review revealed only a single cost-effectiveness study of smoking cessation in the context of cancer, a surgical study conducted in the U.S., which compared the cost-effectiveness of nicotine replacement therapy (NRT) with and without counselling. This study determined that counselling with NRT was cost-effective (21).
The first cost-effectiveness analysis undertaken in an Ontario group used a Markov model and compared the current standard of a basic smoking cessation program (screening, advice, and referral) to a “best practice” program (current standard plus pharmacotherapy, counselling and follow-up) (20). A hypothetical cohort of cancer patients, who were receiving treatment, were followed and assessed for readiness to quit over their lifetime. Transition probabilities, mortality rates and utilities were obtained from the published literature. Cost parameters were obtained from standard Ontario costing sources. Probabilistic and deterministic sensitivity analyses were undertaken. Costs (in 2015 Canadian dollars) and outcomes were discounted at 5%. For smokers with cancer, the best practice smoking cessation program was more effective and costlier than the basic smoking cessation program. However, the incremental cost-effectiveness ratio of the best practice smoking cessation program compared to the basic smoking cessation program was $3,367 per quality-adjusted life year (QALY) gained and $5,050 per life year (LY) gained for males, and $2,050 per QALY gained and $4,100 per LY gained for females. The results were most sensitive to the hazard ratio for mortality of former and current smokers, the probability of quitting smoking through participation in the program, and the smoking-attributable costs. The study results strongly suggested that a best practice smoking cessation program would be a cost-effective option.
Building on the cost-effectiveness analysis of smoking cessation in Ontario’s regional cancer programs, the next analysis constructed a Markov model and followed 65-year-old smokers diagnosed with cancer over a lifetime horizon to examine various smoking cessation strategies compared to no intervention. Specifically, provision of NRT (patch and inhaler), bupropion, varenicline, intensive counseling, and intensive counseling with NRT as standalone strategies were compared to a no intervention strategy. Quit rates for each strategy were obtained from a published meta-analysis (22), and costs were derived from published Canadian sources. Costs and outcomes were discounted at 1.5% annually and were reported in 2017 Canadian dollars. A probabilistic analysis was used as the base case, and model parameters were varied deterministically in sensitivity analysis. The results indicated that almost all strategies were less costly and more effective than no intervention. Weekly intensive counselling with a nurse for 15 minutes over 12 weeks was the most economically attractive option. This strategy cost $1,047 less and produced 0.06 more QALYs compared to no intervention. Results were most sensitive to the hazard ratio of mortality for former and current smokers. From these results, it can be concluded that treatment for smoking cessation in cancer patients is cost-effective compared to no intervention, with intensive counselling being the most favourable option. These completed economic analyses provide support for smoking cessation initiatives within cancer treatment centres.
Conclusions
The substantial immediate and long-term health benefits for cancer patients who quit smoking necessitate that cancer care providers screen all patients for smoking status, advise smokers of the benefits that can be gained from cessation, and help them to access smoking cessation services that ideally include counselling and pharmacotherapy interventions. Studies undertaken in the general population and in hospital-based patients have consistently shown that smoking cessation is a cost-effective intervention, and economic evaluations that have been undertaken in Ontario further suggest that this would also likely be true in the cancer setting. This paper has described the Ontario experience of introducing a smoking cessation initiative across a large jurisdiction with multiple cancer centres of varying size. Key to successful implementation are endorsement by the provincial and regional leaders, the establishment of a secretariat to guide the initiative and to capture performance metrics, and the use of performance management to drive change. Additionally, having ongoing advice from an advisory committee of experts, regional champions highly motivated to promote smoking cessation within the regional cancer centres, and effective educational programs to address the ongoing needs of healthcare providers and patients are critical to success. It is important to minimize the burden on busy oncology practices when introducing a smoking cessation program, but oncologists and other cancer care providers must come to see their role in promoting smoking cessation to their patients as critical to achieving the best clinical outcomes for their patients.
In the near future, the smoking cessation initiative will expand to include additional patient populations. Increased funding will enable regions to flexibly choose expansion to address the needs of cancer patients in partner hospitals or diagnostic assessment programs. Most regions have diagnostic assessment units or programs for lung, breast, colorectal and prostate cancers. Expansion of cessation services to assessment programs will serve as primary prevention for those not found to have cancer and allow earlier efforts to support cessation for those who will require some form of cancer treatment.
In addition, it will be critical to look beyond whether a smoker has accepted a referral to gather information on quit attempts. In the coming year, an effort will be made to capture quit rates at defined intervals using electronic kiosks located in two cancer centres. Information from this pilot project will help determine the effectiveness of the current initiative and give impetus for further program expansion.
Key to all these efforts is the recognition that it is never too late for a cancer patient to quit smoking and that the integration of smoking cessation into all cancer services is a measure of their quality.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Health Consequences of Smoking – 50 years of Progress: A report of the Surgeon General, 2014. Available online http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- Parada H Jr, Sun X, Tse CK, et al. Lifestyle Patterns and Survival Following Breast Cancer in the Carolina Breast Cancer Study. Epidemiology 2019;30:83-92. [Crossref] [PubMed]
- Gild P, Vetterlein M, Gontero P, et al. The impact of cigarette smoking on adverse pathological features and survival in patients undergoing radical cystectomy for urinary bladder cancer - a prospective, European multicenter study of the EAU young academic urologists urothelial carcinoma group. Eur Urol Suppl 2018;17:e1031-e2. [Crossref]
- Minami Y, Kanemura S, Oikawa T, et al. Associations of cigarette smoking and alcohol drinking with stomach cancer survival: A prospective patient cohort study in Japan. Int J Cancer 2018;143:1072-85. [Crossref] [PubMed]
- Japuntich SJ, Kumar P, Pendergast JF, et al. Smoking status and survival among a national cohort of lung and colorectal cancer patients. Nicotine Tob Res 2019;21:497-504. [Crossref] [PubMed]
- Morgan G, Schnoll RA, Alfano CM, et al. National Cancer Institute conference on treating tobacco dependence at cancer centres. J Oncol Pract 2011;7:178-82. [Crossref] [PubMed]
- Croyle RT, Morgan GD, Fiore MC. Addressing a core gap in cancer care – the NCI Moonshot Program to help oncology patients stop smoking. N Engl J Med 2019;380:512-5. [Crossref] [PubMed]
- Warren GW, Marshall JR, Cummings KM, et al. IASLC Tobacco Control and Smoking Cessation Committee. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol 2013;8:543-8. [Crossref] [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [Crossref] [PubMed]
- Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 1993;328:159-63. [Crossref] [PubMed]
- Statistics Canada. Canadian Community Health Survey – annual component (CCHS). Ottawa, ON: Statistics Canada, 2015. Available online: http://www.23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3226
- Liu J, Chadder J, Fung S, et al. Smoking behaviours of current cancer patients in Canada. Curr Oncol 2016;23:201-3. [Crossref] [PubMed]
- Data Book Holdings, February 2019 (Cancer Care Ontario). Available online: https://www.cancercareontario.ca/en/data-book-reporting-standards
- Toll BA, Brandon TH, Gritz ER, et al. AACR Subcommittee on Tobacco and Cancer. Assessing Tobacco Use by Cancer Patients and Facilitating Cessation: An American Association for Cancer Research Policy Statement. Cancer Clin Cancer Res 2013;19:1941-8. [Crossref] [PubMed]
- Evans WK, Truscott R, Cameron E, et al. Lessons learned implementing a province-wide smoking cessation initiative in Ontario’s cancer centres. Curr Oncol 2017;24:e185-90. [Crossref] [PubMed]
- UK Department of Health. NHS Stop Smoking Services: service and monitoring guidance 2010/11. Available online https://webarchive.nationalarchives.gov.uk
- Cromwell J, Bartosch WJ, Fiore MC, et al. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA 1997;278:1759-66. [Crossref] [PubMed]
- Reid RD, Mullen KA, Slovinec D'Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the "Ottawa Model". Nicotine Tob Res 2010;12:11-8. [Crossref] [PubMed]
- Mullen KA, Coyle D, Manuel D, et al. Economic evaluation of a hospital-initiated intervention for smokers with chronic disease in Ontario, Canada. Tob Control 2015;24:489-96. [Crossref] [PubMed]
- Djalalov S, Masucci L, Isaranuwatchai W, et al. Economic evaluation of smoking cessation in Ontario's regional cancer programs. Cancer Med 2018;7:4765-72. [Crossref] [PubMed]
- Slatore CG, Au DH, Hollingworth W. Cost-effectiveness of a smoking cessation program implemented at the time of surgery for lung cancer. J Thorac Oncol 2009;4:499-504. [Crossref] [PubMed]
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008;35:158-76.