Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis
Introduction
Lung cancer is the most commonly diagnosed cancer and a leading cause of cancer death (1). Surgical treatment can be curative, but efficacy is limited to early stage lung cancer (2). The majority of patients are diagnosed with metastatic diseases at the initial visit, which highlights the importance of effective systemic therapies (3). The traditional therapy is chemotherapy, and certain gene expression patterns (4,5) and liquid biomarkers (6,7) serve as predictors of chemotherapy outcomes. Targeted therapy with small molecule tyrosine kinase inhibitors and immunotherapy with immune checkpoint inhibitors has improved patient survival and transformed the treatment paradigm of non-small cell lung cancer (NSCLC). According to the National Comprehensive Cancer Network guideline of NSCLC (Version 3. 2019), targeted therapy is the standard front-line treatment for advanced NSCLC patients with driver mutations, and pembrolizumab is the preferred first-line treatment for programmed cell death protein 1 ligand (PD-L1) expressing advanced NSCLC patients harboring negative driver mutations. The identification of targetable gene alterations can help select patients who may benefit from targeted therapy, whilst PD-L1 expression (8,9) and the tumor mutational burden (TMB) (10-12) are proposed biomarkers for both the response and outcome of immunotherapy.
Neutrophil-to-lymphocyte ratios (NLR), defined as the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC) from whole blood, can be easily and inexpensively accessed from regular blood tests and are associated with the prognosis of various cancers (13,14), including lung cancer (15,16). Our previous study indicated that elevated pretreatment NLR is an independent predictor of inferior survival for NSCLC patients receiving chemotherapy (17), which has been conflicted (18-20) and supported (21-24) by other studies. To our knowledge, no consensus on this relationship has been reached and there are a lack of recent meta-analyses (MAs) that comprehensively assess the relationship between pretreatment NLR and systemic treatment outcomes for NSCLC. We therefore performed an MA by referring to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, to investigate the prognostic role of pretreatment NLR from whole blood during lung cancer systemic therapy, including chemotherapy, immunotherapy and targeted therapy.
Methods
Search strategy and study selection
PubMed, Web of Science and Cochrane Library databases were systematically searched for published studies from the inception of each database to April 09, 2019. No language restrictions were applied. Search terms included “neutrophil”, “lymphocyte”, “ratio”, “NLR”, “dNLR” and “lung cancer”. Reference lists of selected articles were manually explored to ensure a complete literature search.
Reports were considered eligible if they met the following criteria: (I) studies involving NSCLC patients treated with chemotherapy, immunotherapy, targeted therapy, or their combination; (II) studies providing multivariable-adjusted hazard ratios (HR) with 95% confidence intervals (CI) for progression-free survival (PFS) or overall survival (OS), calculated using Cox proportional hazard analyses; and (III) studies assessing NLR at the time before the initiation of systemic therapy.
Exclusion criteria were: (I) studies including patients with other tumor types and in which subgroup analysis according to tumor type was not performed; (II) studies not specifying treatment strategies; (III) studies including patients receiving other types of treatment and subgroup analysis according to treatment strategy was not performed; (IV) studies published as review articles, letters, editorials, comments, or meeting abstracts; or (V) studies containing repeated data and not with the largest sample size or latest information.
Quality assessment
Study quality was assessed using the Newcastle-Ottawa Scale, which evaluated three aspects of the selected studies: selection, comparability and outcome. A maximum of 9 stars could be given for each study. A higher number of stars indicated better study quality.
Data extraction
Data were extracted from reports containing first author’s name, year of publication, region, study design, numbers of enrolled patients, treatment type, NLR cut-off values and length of follow-up. Multivariable-adjusted HRs of each study and corresponding 95% CIs for PFS or OS according to pretreatment NLR were also retrieved.
Statistical analysis
To investigate the relationship between pretreatment NLR and survival outcomes of the NSCLC patients receiving systemic therapy, HRs with 95% CI were pooled to give the effective value. Since the HRs extracted from included studies were estimates of the ratio for higher NLR over lower NLR, a pooled HR >1 indicated inferior survival for the group with elevated pretreatment NLR.
The heterogeneity of the studies was assessed through the Cochrane Q test and I2 statistics. A P<0.05 in the Cochrane Q test and I2>50% were interpreted as significant heterogeneity. A random effects model was used if statistically significant heterogeneity was indicated. A fixed effects model was otherwise applied.
Subgroup analysis stratified by treatment strategy was performed to test if pretreatment NLR could predict survival outcomes in each type of treatment. Subgroup analysis according to NLR cut-off values were also conducted, as various levels of NLR cut-off values were employed. In the study by Maymani et al. (25), the lower level of NLR cut-off failed to predict survival, whilst the higher NLR value could. Studies were allocated into two groups according to median NLR cut-off values of PFS and OS. Subgroup analyses according to study design, region, sample size and methods of cut-off determination were also performed. Publication bias was assessed using funnel plots, Begg’s test and Egger’s test. All calculations were performed by STATA version 12.0 (Stata Corporation, College Station, TX, USA). P values were two-sided and statistical significance was taken as a P<0.05.
Results
Literature search
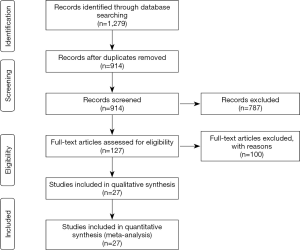
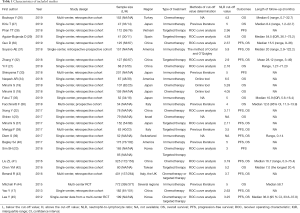
A total of 1,279 records were identified in the literature research. After excluding duplicated records and screening titles and abstracts, 127 records were evaluated by full text and 27 articles (Table 1) with 4,298 patients were selected for final synthesis (Figure 1). One publication (46) was discarded as it included a redundant population (41).
Full table
Study characteristics
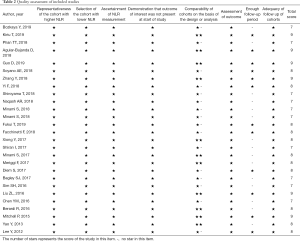
The major characteristics of the included studies are summarized in Table 1. Regarding treatment strategy, 9 articles assessed immunotherapy, 6 articles assessed targeted therapy, 9 articles assessed chemotherapy, 2 articles reported both targeted therapy and chemotherapy, and a single article presented data on targeted therapy and chemotherapy independently. Fifteen reports presented data related to PFS and 24 reports presented data on OS. Regarding study design, 23 reports were retrospective cohort studies, 2 reports were prospective cohort studies and 2 reports provided data from randomized controlled trials. One article (21) was published in Chinese and the rest were all published in English. The quality assessment of the selected studies is shown in Table 2.
Full table
Association between pretreatment NLR and PFS
Fifteen reports with 2,599 patients were chosen for the pooled analysis of the association between pretreatment NLR and PFS. The median value of the NLR cut-off was 3.11 (range, 2.11–5.90). Since significant heterogeneity (I2=77.7%, P=0.00) was indicated, a random effects model was applied. The pooled results (Figure 2) suggested that higher pretreatment NLR was associated with a poorer PFS (HR, 1.45, 95% CI, 1.28–1.66).
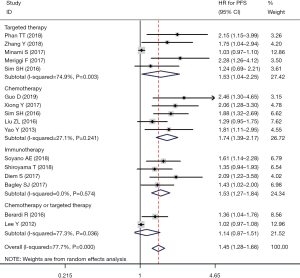
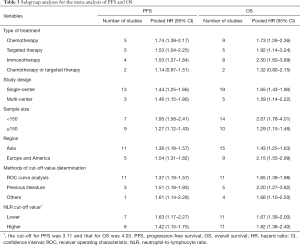
Subgroup analysis according to treatment strategy (Figure 2) showed that the prognostic effects of pretreatment NLR existed in all the systemic therapies, including chemotherapy (HR, 1.74, 95% CI, 1.39–2.17), immunotherapy (HR, 1.53, 95% CI, 1.27–1.84) and targeted therapy (HR, 1.53, 95% CI, 1.04–2.25). Subgroup analysis according to the NLR cut-off values suggested no significant differences between higher (HR, 1.42, 95% CI, 1.15–1.75) and lower NLR cut-off values (HR, 1.63, 95% CI, 1.17–2.27) existed for the prediction of PFS. Subgroup analyses stratified by the study design, region, sample size and methods of cut-off value determination are summarized in Table 3. Significant differences between subgroups were detected in the subgroup analysis of the sample size.
Full table
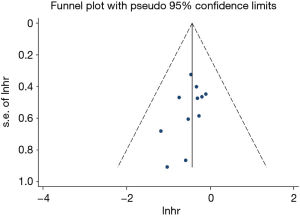
The funnel plot was basically symmetrical (Figure S1) and the results of Begg’s test (P=0.119) and Egger’s test (P=0.149) indicated a lack of publication bias in our pooled analysis.
Association between pretreatment NLR and OS
Twenty-four reports with 3,735 patients were used to analyze the correlation of pretreatment NLR and OS. The median NLR cut-off value was 4.03 (range, 2.11–6.50). A random effects model was adopted due to significant heterogeneity (I2=82.8%, P=0.000). The pooled result (Figure 3) suggested that elevated pretreatment NLR correlated with inferior OS (HR, 1.63, 95% CI, 1.43–1.84).
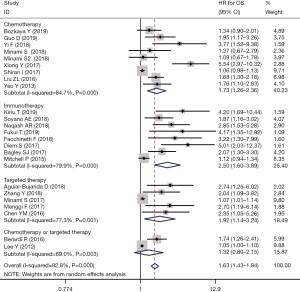
Subgroup analysis according to treatment strategy (Figure 3) indicated that the relationship didn’t markedly change for chemotherapy (HR, 1.73, 95% CI, 1.26–2.36), immunotherapy (HR, 2.50, 95% CI, 1.60–3.89) or targeted therapy (HR, 1.92, 95% CI, 1.14–3.24). Subgroup analysis according to the NLR cut-off values showed that higher (HR 1.82, 95% CI, 1.38–2.40) and lower (HR 1.67, 95% CI, 1.39–2.00) values had a similar ability to predict OS. Subgroup analyses stratified by study design, region, sample size and the methods of cut-off value determination are summarized in Table 3. Similar to the PFS, studies with smaller sample sizes had higher HR values.
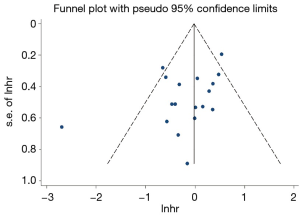
No publication bias was detected following pooled analysis by Begg’s test (P=0.162) or Egger’s test (P=0.056). The funnel plot was almost symmetrical (Figure S2).
Discussion
Inflammation plays an important role in tumorigenesis and development (47) and NLR as a biomarker of inflammation, is associated with treatment outcomes in various types of cancer (48-52). The current MA pooled the results from 27 studies consisting of 4,298 patients and indicated that for NSCLC patients treated with systemic therapy, elevated pretreatment NLR is associated with an inferior survival outcome. In addition, this MA highlighted for the first time that a higher level of pretreatment NLR predicts poorer survival for NSCLC patients receiving targeted therapy.
Previous MAs published in the last years explored the role of pretreatment NLR in lung cancer, mainly focusing on prognosis as opposed to treatment strategy (15,16,53-56). Although subgroup analyses according to treatment strategy were performed in some of the MAs (16,53-56), only chemotherapy was investigated in terms of systemic therapy (15,53,55) and no consensus on the association of pretreatment NLR and the survival outcomes of chemotherapy were achieved. The validity of the results was limited as some of the patients were not solely administered chemotherapy. Our MA selected studies in which patients were treated with chemotherapy alone and only the results from multivariate analysis were included to reduce bias. Also, we added studies published in recent years and applied a more comprehensive search strategy to minimize the risk of missing relevant studies. Our results suggested that elevated pretreatment NLR correlated with inferior survival of NSCLC patients receiving chemotherapy.
Immunotherapy, mainly immune checkpoint inhibitors, leads to variable responses in an array of cancers, but only a minority of patients show benefits. Thus, predictors of the response to immunotherapy are urgently required to select appropriate patients that will benefit from this therapy. The levels of PD-L1 expression (9), TMB (10-12) and other markers (57) have been proposed for lung cancer, but no gold standard has been achieved. Several recent MAs assessed the prognostic role of pretreatment NLR in immunotherapy (48,51,58) and suggested that pretreatment NLR was a promising predictive biomarker for cancer patients treated with immunotherapy. When stratified by cancer type to explore this relationship in lung cancer, the data was limited and the MAs failed to reach a consensus. A recent MA (59) focusing on lung cancer showed that higher pretreatment NLR was significantly associated with a poorer PFS and OS for lung cancer patients treated with nivolumab. In this MA, the treatments were not limited to nivolumab. Our results also favored the prognostic role of pretreatment NLR in immunotherapy, predominantly identified in studies that administered nivolumab. Future studies are required to validate our results in lung cancer patients receiving immunotherapy with nivolumab and other drugs.
Of note, different NLR cut-off values were adopted and the selection and source of sources of the cut-off values varied, including receiver operating characteristic (ROC) curve analysis, previously published studies and website tools. The study by Maymani et al. (25) found that different cut-off values showed different efficacies of predicting the treatment outcome. However, our MA indicated that different cut-off values did not significantly alter the association between NLR and survival outcomes, which were consistent with previous MAs (13-16,48,53,54,56,58). The study by Cho et al. (60) showed that in head and neck squamous cell carcinoma, significant HR of OS could be produced by all NLR cut-off values from 2 to 6, suggesting a three-tier classification system (<2, 2 to 6, and ≥6). Similar studies are required to explore the association of pretreatment NLR cut-off values and their prognostic efficacy, and to determine the optimal pretreatment NLR cut-off value in NSCLC as a prognostic tool in clinical practice.
Other tools have been developed to predict the treatment outcomes of cancer patients. A derived NLR (dNLR), defined as the ANC divided by the difference between white blood cell (WBC) counts and ANC, was calculated since only ANC and WBC were recorded in some of the clinical studies. A similar prognostic value to the NLR was observed (61). The dNLR had been assessed as a predictor of treatment outcomes in other tumors receiving immunotherapy (62) or chemotherapy (63,64). In lung cancer, dNLR was a prognostic biomarker of the immunotherapy (65) and chemotherapy (22) outcome. Besides dNLR, prognostic tools integrating some items are also under investigation, including tumor immune dysfunction and exclusion (66), lung immune prognostic index (65), and the Glasgow prognostic score (67).
To our knowledge, this MA is the first to comprehensively assess the association of pretreatment NLR with systemic treatment outcomes for NSCLC. However, several limitations remain. Firstly, the observational design of the included studies may introduce bias to the MA, but we tried to reduce bias through the inclusion of multivariable results. Secondly, because studies on targeted therapy focused on tyrosine kinase inhibitors of the epidermal growth factor receptor (EGFR), we could not assess the relationship of NLR and targeted therapy for other driver mutations. Thirdly, the heterogeneity across studies which may have resulted from different baseline characteristics of the patients, may influence the interpretation of our results.
Conclusions
Elevated pretreatment NLR is associated with inferior survival for NSCLC patients treated with systemic therapy, including chemotherapy, immunotherapy and targeted therapy. Although higher and lower pretreatment NLR cut-off values have a similar ability to predict survival, further studies are required to determine the optimal cut-off values. Future clinical trials are warranted to decide whether pretreatment NLR should be incorporated into the prognostic tools of lung cancer patients, to identify those most likely to benefit from systemic therapies.
Acknowledgments
We sincerely thank all authors, investigators, sponsors and patients for their effort and participation in our included studies.
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81401903, 81572937 and 81572273); the Natural Science Foundation of Jiangsu province (grant number BK20180139 and BK20161386); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), the Jiangsu Provincial Key Research and Development Program (No. BE2016721).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-24. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Li YQ, Chen J, Yin JY, et al. Gene expression and single nucleotide polymorphism of ATP7B are associated with platinum-based chemotherapy response in non-small cell lung cancer patients. J Cancer 2018;9:3532-9. [Crossref] [PubMed]
- Chen HH, Yan JJ, Chen WC, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer 2012;75:228-34. [Crossref] [PubMed]
- Jin J, Si J, Liu Y, et al. Elevated serum soluble programmed cell death ligand 1 concentration as a potential marker for poor prognosis in small cell lung cancer patients with chemotherapy. Respir Res 2018;19:197. [Crossref] [PubMed]
- Yuwen D, Ma Y, Wang D, et al. Prognostic role of circulating exosomal miR-425-3p for the response of NSCLC to platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev 2019;28:163-73. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2019;35:329. [Crossref] [PubMed]
- Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018;33:843-52.e4. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev 2017;58:1-13. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Yang HB, Xing M, Ma LN, et al. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget 2016;7:76769-78. [Crossref] [PubMed]
- Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Mol Clin Oncol 2017;7:498-506. [Crossref] [PubMed]
- Yao Y, Yuan D, Liu H, et al. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother 2013;62:471-9. [Crossref] [PubMed]
- Minami S, Ihara S, Kim SH, et al. Lymphocyte to Monocyte Ratio and Modified Glasgow Prognostic Score Predict Prognosis of Lung Adenocarcinoma Without Driver Mutation. World J Oncol 2018;9:13-20. [Crossref] [PubMed]
- Minami S, Ihara S, Komuta K. Pretreatment Lymphocyte to Monocyte Ratio as a Prognostic Marker for Advanced Pulmonary Squamous Cell Carcinoma Treated With Chemotherapy. J Clin Med Res 2018;10:657-64. [Crossref] [PubMed]
- Shiran I, Heller E, Jessel S, et al. Non-Small-cell Lung Cancer Patients With Adenocarcinoma Morphology Have a Better Outcome Compared With Patients Diagnosed With Non-Small-cell Lung Cancer Favor Adenocarcinoma. Clin Lung Cancer 2017;18:316-23.e1. [Crossref] [PubMed]
- Yi F, Gu Y, Chen S, et al. Zhongguo Fei Ai Za Zhi 2018;21:481-92. [Impact of the Pretreatment or Posttreatment NLR and PLR on the Response of First Line Chemotherapy and the Outcomes in Patients with Advanced Non-small Cell Lung Cancer]. [PubMed]
- Xiong Y, Zhao N, Zheng Y, et al. Prognostic value of pretreatment inflammatory biomarkers in advanced lung adenocarcinoma patients receiving first-line pemetrexed/platinum doublet. Tumour Biol 2017;39:1010428317701639. [Crossref] [PubMed]
- Sim SH, Beom SH, Ahn YO, et al. Pretreatment neutrophil-lymphocyte ratio is not a significant prognostic factor in epidermal growth factor receptor-mutant non-small cell lung cancer patients treated with tyrosine kinase inhibitors. Thorac Cancer 2016;7:161-6. [Crossref] [PubMed]
- Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950-8. [Crossref] [PubMed]
- Maymani H, Hess K, Groisberg R, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer 2018;120:137-41. [Crossref] [PubMed]
- Bozkaya Y, Kurt B, Gürler F. A prognostic parameter in advanced non-small cell lung cancer: the ratio of hemoglobin-to-red cell distribution width. Int J Clin Oncol 2019;24:798-806. [Crossref] [PubMed]
- Kiriu T, Yamamoto M, Nagano T, et al. Prognostic Value of Red Blood Cell Distribution Width in Non-small Cell Lung Cancer Treated with Anti-programmed Cell Death-1 Antibody. In Vivo 2019;33:213-20. [Crossref] [PubMed]
- Phan TT, Ho TT, Nguyen HT, et al. The prognostic impact of neutrophil to lymphocyte ratio in advanced non-small cell lung cancer patients treated with EGFR TKI. Int J Gen Med 2018;11:423-30. [Crossref] [PubMed]
- Aguiar-Bujanda D, Dueñas-Comino A, Saura-Grau S, et al. Neutrophil to Lymphocyte Ratio as a Prognostic Factor in European Patients with Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Treated with Tyrosine Kinase Inhibitors. Oncol Res Treat 2018;41:755-61. [Crossref] [PubMed]
- Guo D, Li M, Chen D, et al. Neutrophil-to-lymphocyte ratio is superior to platelet-to-lymphocyte ratio as a prognostic predictor in advanced non-small-cell lung cancer treated with first-line platinum-based chemotherapy. Future Oncol 2019;15:625-35. [Crossref] [PubMed]
- Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung Cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer 2018;6:129. [Crossref] [PubMed]
- Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine (Baltimore) 2018;97:e11648. [Crossref] [PubMed]
- Shiroyama T, Suzuki H, Tamiya M, et al. Clinical Characteristics of Liver Metastasis in Nivolumab-treated Patients with Non-small Cell Lung Cancer. Anticancer Res 2018;38:4723-9. [Crossref] [PubMed]
- Naqash AR, Stroud CRG, Butt MU, et al. Co-relation of overall survival with peripheral blood-based inflammatory biomarkers in advanced stage non-small cell lung cancer treated with anti-programmed cell death-1 therapy: results from a single institutional database. Acta Oncol 2018;57:867-72. [Crossref] [PubMed]
- Fukui T, Okuma Y, Nakahara Y, et al. Activity of Nivolumab and Utility of Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study. Clin Lung Cancer 2019;20:208-14.e2. [Crossref] [PubMed]
- Facchinetti F, Veneziani M, Buti S, et al. Clinical and hematologic parameters address the outcomes of non-small-cell lung cancer patients treated with nivolumab. Immunotherapy 2018;10:681-94. [Crossref] [PubMed]
- Minami S, Ogata Y, Ihara S, et al. Neutrophil-to-Lymphocyte Ratio Predicts Overall Survival of Advanced Non-Small Cell Lung Cancer Harboring Mutant Epidermal Growth Factor Receptor. World J Oncol 2017;8:180-7. [Crossref] [PubMed]
- Meriggi F, Codignola C, Beretta GD, et al. Significance of neutrophil-to-lymphocyte ratio in Western advanced EGFR-mutated non-small cell lung cancer receiving a targeted therapy. Tumori 2017;103:443-8. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]
- Liu ZL, Zeng TT, Zhou XJ, et al. Neutrophil-lymphocyte ratio as a prognostic marker for chemotherapy in advanced lung cancer. Int J Biol Markers 2016;31:e395-401. [Crossref] [PubMed]
- Chen YM, Lai CH, Rau KM, et al. Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2016;16:868. [Crossref] [PubMed]
- Berardi R, Rinaldi S, Santoni M, et al. Prognostic models to predict survival in patients with advanced non-small cell lung cancer treated with first-line chemo- or targeted therapy. Oncotarget 2016;7:26916-24. [Crossref] [PubMed]
- Mitchell P, Thatcher N, Socinski MA, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol 2015;26:1134-42. [Crossref] [PubMed]
- Lee Y, Kim SH, Han JY, et al. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol 2012;138:2009-16. [Crossref] [PubMed]
- Liu D, Jin J, Zhang L, et al. The Neutrophil to Lymphocyte Ratio May Predict Benefit from Chemotherapy in Lung Cancer. Cell Physiol Biochem 2018;46:1595-605. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother 2018;67:713-27. [Crossref] [PubMed]
- Mellor KL, Powell A, Lewis WG. Systematic Review and Meta-Analysis of the Prognostic Significance of Neutrophil-Lymphocyte Ratio (NLR) After R0 Gastrectomy for Cancer. J Gastrointest Cancer 2018;49:237-44. [Crossref] [PubMed]
- Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 2014;23:31-9. [Crossref] [PubMed]
- Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther 2018;11:955-65. [Crossref] [PubMed]
- Zheng J, Cai J, Li H, et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem 2017;44:967-81. [Crossref] [PubMed]
- Zhao QT, Yang Y, Xu S, et al. Prognostic role of neutrophil to lymphocyte ratio in lung cancers: a meta-analysis including 7,054 patients. Onco Targets Ther 2015;8:2731-8. [PubMed]
- Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524-30. [Crossref] [PubMed]
- Peng B, Wang YH, Liu YM, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med 2015;8:3098-106. [PubMed]
- Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015;5:12493. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Tan Q, Liu S, Liang C, et al. Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: A meta-analysis. Thorac Cancer 2018;9:1220-30. [Crossref] [PubMed]
- Cao D, Xu H, Xu X, et al. A reliable and feasible way to predict the benefits of Nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology 2018;7:e1507262. [Crossref] [PubMed]
- Cho JK, Kim MW, Choi IS, et al. Optimal cutoff of pretreatment neutrophil-to-lymphocyte ratio in head and neck cancer patients: a meta-analysis and validation study. BMC Cancer 2018;18:969. [Crossref] [PubMed]
- Proctor MJ, McMillan DC, Morrison DS, et al. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 2012;107:695-9. [Crossref] [PubMed]
- Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732-8. [Crossref] [PubMed]
- Grenader T, Nash S, Plotkin Y, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol 2015;26:1910-6. [Crossref] [PubMed]
- van Soest RJ, Templeton AJ, Vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol 2015;26:743-9. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 2018;24:1550-8. [Crossref] [PubMed]
- Jiang AG, Lu HY. The Glasgow prognostic score as a prognostic factor in patients with advanced non-small cell lung cancer treated with cisplatin-based first-line chemotherapy. J Chemother 2015;27:35-9. [Crossref] [PubMed]