
Carcinoembryonic antigen and CYFRA 21-1 responses as prognostic factors in advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) is the most common histological type of lung cancer; more than half of these tumors are unresectable at the time of diagnosis (2). With the development of precision medicine for advanced NSCLC, various tyrosine kinase inhibitors (TKIs) can be prescribed in addition to conventional cytotoxic chemotherapy regimens. Despite the increase in treatment options, NSCLC still has a poor prognosis.
Multiple prognostic factors in NSCLC such as TNM stage, histology, age, performance status, progression-free survival (PFS) (3-6), and serum tumor marker levels were reported (7,8). Patients with advanced NSCLC usually undergo follow-up imaging with computed tomography (CT) after first-line chemotherapy; second-line therapy is prescribed upon diagnosis with disease progression as determined by the Response Evaluation Criteria in Solid Tumors (RECIST) (9). However, the RECIST guidelines are not adequate in patients with multiple metastases and/or non-measurable disease.
Serum tumor markers are widely used in the diagnosis and monitoring of NSCLC (10-12), with carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA 21-1) being most sensitive (13-16). Both were reported as prognostic factors for overall survival (OS) in patients with resected NSCLC (7,8). However, another study reported controversial findings on whether serum CEA was associated with OS in NSCLC (17). Moreover, the significance of changes in serum CEA and CYFRA 21-1 in response to treatment has not been clarified.
We investigated the association between the treatment responses of CEA and CYFRA 21-1 and OS of patients with NSCLC.
Methods
Patients
We retrospectively examined treatment-naïve patients with advanced or relapsed NSCLC, European Cooperative Oncology Group Performance Status (ECOG PS) 0, 1 or 2, and increased serum CEA or CYFRA 21-1 who were treated at the Japanese Red Cross Kyoto Daiichi Hospital between April 2010 and December 2015. All patients who receive chemotherapy in our hospital quit smoking at the start of treatment. The eligibility criteria were: (I) a pathological NSCLC diagnosis; (II) platinum-based doublet therapy for ≥4 cycles or epidermal growth factor receptor (EGFR) TKIs for ≥4 months as first-line treatment; and (III) measurement of serum CEA and CYFRA 21-1 levels at 1 and 4 months after treatment initiation.
This study was approved by the institutional review board of the Japanese Red Cross Kyoto Daiichi Hospital (no. 666) and conducted in accordance with the principles of the Declaration of Helsinki.
Tumor marker responses
CEA and CYFRA 21-1 levels in sera obtained from peripheral venous blood were measured by a radioimmunoassay (cobas® 8000, Roche Diagnostic K.K., Tokyo, Japan and Chemiluminescent Enzyme Immunoassay System LUMIPULSE® L2400, FUJIREBIO INC., Tokyo, Japan). Pretreatment serum CEA and CYFRA 21-1 levels ≥5 and ≥3.5 ng/mL, respectively, were defined as positive. Patients with a decrease in serum tumor marker levels of greater than 25% compared to pretreatment levels were categorized as the “decreasing group” and all others as the “non-decreasing group,” based on a previous report (18).
Imaging-based tumor responses
Imaging-based responses (IBRs) were evaluated based on the RECIST using CT and categorized as a complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (9).
Statistical analysis
Comparisons between the two groups were performed using the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. OS was analyzed with the Kaplan-Meier method and compared with the log-rank test. Prognostic factors for OS were identified using univariate and multivariate analyses. Multivariate analysis was performed with the factors found to be significant in the univariate analysis and PS. P values less than 0.05 were considered significant. All statistical analyses were performed with Prism (version 8.01; GraphPad Software Inc, CA, USA).
Results
Patient characteristics
A total of 748 patients diagnosed with advanced NSCLC were analyzed, including 96 and 55 CEA- and CYFRA 21-1-positive patients, respectively (Figure 1). Among CEA-positive patients, 74 (77.9%) and 79 (82.2%) were in the decreasing group at 1 and 4 months, respectively. Among CYFRA 21-1-positive patients, 47 (85.5%) and 39 (70.9%) were in the decreasing group at 1 and 4 months, respectively. There were measurable lesions in 86 and 50 patients and non-measurable lesions in 10 and 5 patients who were positive for CEA and CYFRA 21-1, respectively. While there was no significant difference between the tumor marker response (TMR) groups at 4 months, there were significantly more patients who were female, never smoked, and responded to chemotherapy in the decreasing group (Table 1).
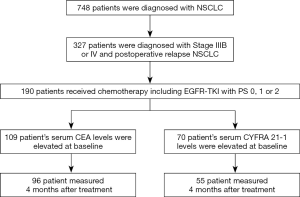
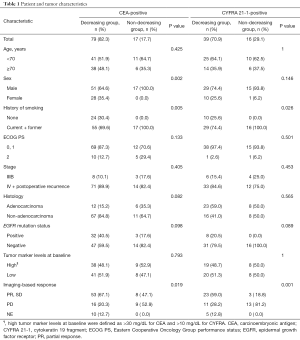
Full table
OS as a function of TMR
In CEA-positive patients, there was no significant difference in OS between the decreasing and non-decreasing groups 1 month post-treatment initiation (16.9 vs. 13.4 months, P=0.684) (Figure 2A). Median OS at 4 months post-treatment initiation was significantly different between the two groups [16.9 vs. 10.3 months; hazard ratio (HR), 0.50; 95% confidence interval (CI), 0.27–0.93; P=0.025] (Figure 2B).
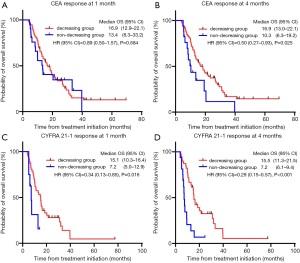
In CYFRA 21-1-positive patients, the decreasing group showed a significantly longer OS at 1 and 4 months post-treatment initiation than the non-decreasing group. Median OS in the decreasing and non-decreasing groups at 1 month post-treatment initiation was 15.1 and 7.2 months, respectively (HR, 0.34; 95% CI, 0.13–0.85; P=0.016) and 15.5 and 7.2 months, respectively, at 4 months post-treatment initiation (HR, 0.29; 95% CI, 0.15–0.57; P<0.001) (Figure 2C,D).
OS according to image-based responses
CT at 4 months post-treatment initiation was performed in 86 and 50 CEA- and CYFRA 21-1-positive patients, respectively (i.e., those with measurable lesions). Among CEA-positive patients, 0 (0%), 40 (46.5%), 21 (24.4%), and 25 (29.1%) exhibited a CR, PR, SD, and PD, respectively. Among CYFRA 21-1-positive patients, 0 (0%), 19 (38.0%), 7 (14.0%), and 24 (48.0%) exhibited a CR, PR, SD, and PD, respectively.
Predictors for survival
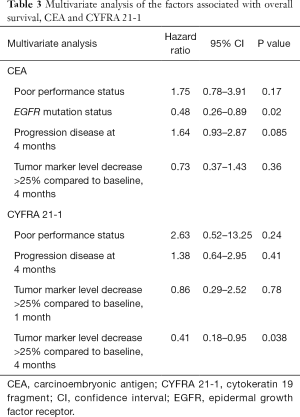
Univariate analysis identified EGFR mutation status, IBR, and CEA response at 4 months as predictors of OS in the CEA-positive group. ECOG PS, IBR, and CYFRA 21-1 response at 1 and 4 months were predictors of OS in the CYFRA 21-1-positive group (Table 2). In the multivariate analysis, EGFR mutation status in the CEA-positive group and serum CYFRA 21-1 response at 4 months in the CYFRA 21-1-positive group were significantly associated with OS (HR, 0.48 and 0.41; 95% CI, 0.26–0.89 and 0.18–0.95; P=0.02 and P=0.038, respectively) (Table 3).
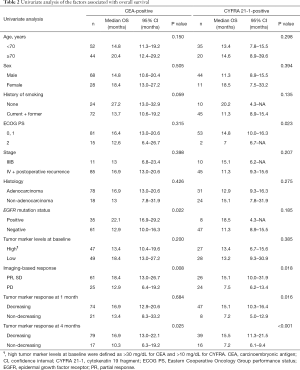
Full table
Full table
Discussion
In this study, a reduction of greater than 25% in serum CEA or CYFRA 21-1 levels at 4 months after chemotherapy was significantly associated with a longer OS in patients with advanced NSCLC. In addition, the reduction in serum CYFRA 21-1 levels at 4 months was independent of ECOG PS and the IBR.
In a meta-analysis of patients with advanced NSCLC enrolled in phase III trials, the IBR (i.e., objective response and disease control rates) after first-line chemotherapy was an independent prognostic factor (19). Although the IBR according to the RECIST guidelines is a well-established method of tumor evaluation, it is not necessarily accurate. The RECIST guidelines include the evaluation of the sum of the longest diameter of up to 5 target lesions (9). In advanced NSCLC, however, the total tumor burden, and changes therein, are not evaluated properly with the IBR because there may be multiple metastatic lesions, some of which may be undetectable. Even a measurable lesion cannot be evaluated adequately if tumor necrosis, hemorrhage, and/or cavitation are present.
The associations between tumor marker expression levels and prognosis have been investigated previously. Although a meta-analysis showed that CYFRA 21-1 was a prognostic factor in NSCLC (8), another study reported controversial findings for CEA (17). In this study, we found no significant association between serum tumor markers at baseline and OS. A previous study found that CEA and CYFRA 21-1 levels correlated well with the tumor volume in patients with resectable NSCLC (7,8), suggesting that serum tumor marker levels drop when tumors shrink following chemotherapy.
Here, we demonstrated that the responses of serum tumor markers at 4 months, especially for CYFRA 21-1, might be a good predictive factor, among several factors, including the IBR, in patients with advanced NSCLC and positive tumor marker levels at baseline. Only a decrease in serum CYFRA 21-1, but not serum CEA, was associated with a longer OS at 1 month post-treatment initiation. A previous study showed that CA125 and CA19-9 levels at 4 weeks post-treatment initiation were independent prognostic factors only in EGFR mutation-positive NSCLC treated with gefitinib and having more than 25% changes in serum CEA (18); a decrease in serum CYFRA 21-1, but not CEA, at baseline predicted the response to chemotherapy in patients with NSCLC (20); these data are consistent with our results. Although a low-level elevation of serum CEA is observed in smokers, all patients who receive chemotherapy in our hospital quit smoking. Therefore, we anticipated that the impact of smoking on the serum CEA level would not be as large in our study.
In the present study, EGFR mutation status in CEA-positive patients was an independent prognostic factor in the multivariate analysis. In a previous study, EGFR mutation status in advanced NSCLC predicted OS and PFS (21). In lung cancer, patients with an oncogenic driver mutation such as EGFR achieved prolonged survival when receiving targeted therapy (22). The EGFR mutation status might have been an independent prognostic factor in CEA-positive patients only because this mutation is associated with serum CEA, but not serum CYFRA 21-1 levels (23). Although our study population was limited to patients with positive serum tumor markers at baseline, the TMR at 4 months may identify patients with a poor prognosis, leading to improved treatment optimization.
This study has some limitations. First, it had a retrospective design and a relatively small sample size. Second, we evaluated the TMR relative to baseline levels, which may have exaggerated the outcomes in patients with low tumor marker levels; however, this is unlikely because the absolute serum tumor marker levels at diagnosis were not associated with OS. Although patients with only non-measurable lesion are rare, we should evaluate the effect of chemotherapy to determine the best treatment for those patients. Since serum tumor marker response might be a better predictor for several factors, further prospective investigations are warranted to verify our findings.
In conclusion, significant reductions in serum CEA and CYFRA 21-1 levels at 4 months after initiating chemotherapy and/or targeted therapy with EGFR TKIs were positive prognostic indicators in patients with advanced NSCLC with elevated serum tumor marker levels at baseline.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of the Japanese Red Cross Kyoto Daiichi Hospital (no. 666) and conducted in accordance with the principles of the Declaration of Helsinki. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer 2015;87:193-200. [Crossref] [PubMed]
- Woodard GA, Jones KD, Jablons DM. Lung Cancer Staging and Prognosis. Cancer Treat Res 2016;170:47-75. [Crossref] [PubMed]
- Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:357-64. [Crossref] [PubMed]
- Merrill RM, Henson DE, Barnes M. Conditional survival among patients with carcinoma of the lung. Chest 1999;116:697-703. [Crossref] [PubMed]
- Mandrekar SJ, Qi Y, Hillman SL, et al. Endpoints in phase II trials for advanced non-small cell lung cancer. J Thorac Oncol 2010;5:3-9. [Crossref] [PubMed]
- Wang XB, Li J, Han Y. Prognostic significance of preoperative serum carcinoembryonic antigen in non-small cell lung cancer: a meta-analysis. Tumour Biol 2014;35:10105-10. [Crossref] [PubMed]
- Yu Z, Zhang G, Yang M, et al. Systematic review of CYFRA 21-1 as a prognostic indicator and its predictive correlation with clinicopathological features in Non-small Cell Lung Cancer: A meta-analysis. Oncotarget 2017;8:4043-50. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Molina R, Auge JM, Bosch X, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer: correlation with histology. Tumour Biol 2009;30:121-9. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993;270:943-7. [Crossref] [PubMed]
- Molina R, Jo J, Zanon G, et al. Utility of C-erbB-2 in tissue and in serum in the early diagnosis of recurrence in breast cancer patients: comparison with carcinoembryonic antigen and CA 15.3. Br J Cancer 1996;74:1126-31. [Crossref] [PubMed]
- Plebani M, Basso D, Navaglia F, et al. Clinical evaluation of seven tumour markers in lung cancer diagnosis: can any combination improve the results? Br J Cancer 1995;72:170-3. [Crossref] [PubMed]
- Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer. Anticancer Res 1999;19:3613-8. [PubMed]
- Viñolas N, Molina R, Galan MC, et al. Tumor markers in response monitoring and prognosis of non-small cell lung cancer: preliminary report. Anticancer Res 1998;18:631-4. [PubMed]
- Niho S, Nishiwaki Y, Goto K, et al. Significance of serum pro-gastrin-releasing peptide as a predictor of relapse of small cell lung cancer: comparative evaluation with neuron-specific enolase and carcinoembryonic antigen. Lung Cancer 2000;27:159-67. [Crossref] [PubMed]
- Hatzakis KD, Froudarakis ME, Bouros D, et al. Prognostic value of serum tumor markers in patients with lung cancer. Respiration 2002;69:25-9. [Crossref] [PubMed]
- Chiu CH, Shih YN, Tsai CM, et al. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer 2007;57:213-21. [Crossref] [PubMed]
- Hotta K, Fujiwara Y, Kiura K, et al. Relationship between response and survival in more than 50,000 patients with advanced non-small cell lung cancer treated with systemic chemotherapy in 143 phase III trials. J Thorac Oncol 2007;2:402-7. [Crossref] [PubMed]
- Pang L, Wang J, Jiang Y, et al. Decreased levels of serum cytokeratin 19 fragment CYFRA 21-1 predict objective response to chemotherapy in patients with non-small cell lung cancer. Exp Ther Med 2013;6:355-60. [Crossref] [PubMed]
- Hotta K, Kiura K, Toyooka S, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol 2007;2:632-7. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Feng Y, Zhou J, Chen S, et al. Relationship between EGFR gene mutation and serum tumor biomarkers in advanced lung adenocarcinoma. Int J Clin Exp Pathol 2016;9:250-5.


