EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases
Introduction
Non-small cell lung cancer (NSCLC), representing 85% of all lung cancer, was characterized by high incidence of brain metastasis (BM) with approximately 20–40% patients developing BM during the disease course (1-3). Studies reported that BM was a major cause of deaths in NSCLC patients and showed a median overall survival (OS) time about 3–6 months when left untreated (1,4). Historically, the traditional treatment strategies for BM included surgery, radiotherapy alone including whole brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) or combined with systemic therapy such as chemotherapy (5). And the prognosis of NSCLC patients with BM still remains dismal, with a median OS about 4.5 months after WBRT and 7.0 months after active chemotherapy (6,7).
With the discovery of epidermal growth factor receptor (EGFR) abnormality presented in NSCLC patients and subsequently the great efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) shown in the treatment of advanced NSCLC patients harboring EGFR mutation, EGFR-TKIs became the first-line therapy of EGFR mutated advanced NSCLC patients (8). A study found that EGFR mutated NSCLC patients had a higher rate of BM and EGFR mutation subtype was related to the number of BM (9). Later on, EGFR-TKIs was demonstrated to be safe and significantly efficacious in EGFR mutated patients with BM, with an increasing median progression-free survival time (14.5 months) and median overall survival time (21.9 months) (10). In addition, a randomized, phase III study conducted on EGFR mutant patients with multiple BMs (BRAIN) showed that median iPFS was much better in icotinib group than in whole brain irradiation plus chemotherapy group (10.0 versus 4.8 months) (11), which suggested that EGFR-TKIs might be a rational first-line therapeutic option for this specific population. However, several investigations considered that the efficacy of EGFR-TKIs may be abrogated due to reasons as follows: firstly, the existence of the blood-brain-barrier (BBB), which affected the penetration of drugs to the CNS, would finally lead to low concentration of EGFR-TKIs in the cerebrospinal fluid (CSF), then the requirement of higher dose of EGFR-TKIs may resulted in the occurrence of dose-escalation toxicity (12); secondly, the potential heterogeneity of EGFR mutation status between the primary tumor and metastatic site may also impeded the treatment efficacy (13).
A recent research demonstrated that EGFR mutated patients were more sensitive to radiotherapy (14). Similar result had been also founded by Das et al., which showed in vitro that NSCLC cell lines harboring mutations in the tyrosine kinase domain (TKD) of EGFR exhibited a predominantly radiosensitive through incomplete double strand break (DSB) repair, failure to halt DNA synthesis or mitosis (15). Previous studies have confirmed that radiation increased EGFR expression in cancer cells, and the blockage of EGFR signaling pathway by EGFR-TKIs was able to re-sensitize cancer cell to radiotherapy again (16). Moreover, it has been reported that combining WBRT with EGFR-TKIs could not only improve the penetration of gefitinib into CSF via disrupting BBB but also increased the BBB permeability of gefitinib in accordance with escalated dose of WBRT (17).
To achieve better clinical outcome, some preclinical trials had begun to prescribe combination therapy of EGFR-TKIs and brain RT for NSCLC patients with EGFR mutation and BM, and found that combined therapy was well tolerated and showed a synthetic effect on tumor control with a favorable objective response rate (ORR) of approximately 80% patients (18,19). Moreover, a meta-analysis by Jiang et al. further suggested that the combined therapy presented superior response rate and disease control rate (DCR), as well as a markedly prolonged time to central nervous system progression (CNS-TTP) and OS of NSCLC patients with BM, compared with brain RT alone (20). Nevertheless, whether the combination of EGFR-TKIs and brain RT was better than EGFR-TKIs alone in the management of EGFR mutated NSCLC patients with BM still remains controversial. In this study, we aim to explore the optimal strategy for NSCLC patients harboring EGFR mutation and BM, and further figure out the dominant population of the optimal therapy.
Methods
Literature search
Two authors (X Xia and M Guo) independently conducted a comprehensive systematic literature search of online database including PubMed, Embase, Web of Science, and Cochrane library, Medline and Google Scholar, from January 2013 to March 2018 to identify all published randomized controlled trials (RCTs) and observational studies. Searches were limited to human studies, with language restriction only in English. The search terms and relative variants were as follows: EGFR-TKIs, erlotinib, gefitinib, icotinib, afatinib, osimertinib, radiotherapy, whole brain radiation therapy, WBRT, stereotactic radio surgery, SRS, non-small cell lung cancer, NSCLC, brain metastasis (metastases). We also reviewed the references of included articles and related systematic reviews to identify additional studies. All the search results were evaluated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Study selection and quality assessment
The eligible studies should meet the following criteria: (I) study population: EGFR mutant NSCLC patients with BM at the first diagnosis; (II) intervention: EGFR-TKI plus radiotherapy vs. EGFR-TKI alone; (III) study design: RCTs or observational studies including cohort studies; (IV) outcomes measures: at least one outcome reported among the primary outcomes [overall survival (OS) or intra-cranial progression free survival (iPFS)].
The exclusion criteria included: studies were excluded if they were abstracts, case reports, non-comparative studies, reviews and meta-analysis, as well as commentary articles. In addition, studies were excluded if they included patients without information about EGFR status which we think would affect the reliability and accuracy of results. When duplicated publications were identified, we included the most thorough and recent article describing the up-to-date data of the trial. In cases where only the meeting abstract was available and the article was not yet published, we used the data in the abstracts supplemented by other associated materials, including posters and presentation slides, and tried to obtain additional unpublished data by contacting the authors. New-Ottawa scale was used to evaluate the quality of the included studies.
Data extraction
Data extraction was conducted independently by two investigators (K Dong. and M Guo). We recorded all available information, including baseline characteristics of patients, the treatment outcomes (OS and iPFS). If the statistical variables were not directly reported in the article, we calculated them from the available numerical data according to the methods described by Tierney (21). The data from Kaplan-Meier survival curves were read using an Engauge Digitizer version 4.1 (http://engauge-digitizer.software.informer.com/4.1/) to reduce variability.
Statistical analysis
The relative effect of different arms (EGFR-TKIs + brain RT vs. EGFR-TKIs) in terms of OS and iPFS was presented as hazard ratio (HR) and 95% confident interval (CI). The significance of the HR was assessed by the Z test, along with 95% CIs. Statistical heterogeneity was assessed by visual inspection of forest plots, by performing the Chi-square test (assessing the P value), and by calculating the inconsistency index (I2 statistic) (22). Study-level data were pooled using a random effect model in case of any potential bias. Meta-regression was conducted to screening for potential source of heterogeneity, using the proportion of each phenotype as a candidate factor. Subgroup analysis and sensitivity analysis were performed to explore the source of identified heterogeneity if required. Publication bias was estimated by visually assessing the asymmetry of an inverted funnel plot. STATA 13.0 (Stata Corporation, College Station, TX) and Revman 5.3 were used for calculation. Significance was defined as a P-value <0.05.
Results
Study selection
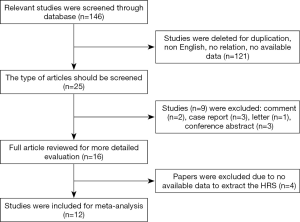
According to the primary searching strategy, a total of 146 potentially eligible articles were displayed. After comparing and skimming titles and abstracts, 121 articles were eliminated due to duplication, no relation or no available data. Then, 9 papers were excluded by screening the type of article, including 2 comment, 3 case report, 1 letter and 3 conference abstracts. 16 candidates were fully reviewed and finally 12 papers meeting inclusive criteria and were selected for meta-analysis (23-34). The flow diagram of our literature inclusion scheme was shown in Figure 1.
Characteristics of the included studies
The pooled analysis enrolled 12 studies. A total of 1,553 BM EGFR mutated NSCLC patients were available for analysis, including 763 patients received EGFR-TKIs plus brain RT (predominantly whole brain RT) and 790 patients received EGFR-TKIs alone. The features of each eligible study were extracted (Table 1). All studies were retrospective studies except one. EGFR-TKI included erlotinib, gefitinib, icotinib, afatinib had been used. The method of detecting the EGFR status is amplification refractory mutation system (ARMS) based on the paraffin section or polymerase chain reaction amplification. Most patients of the included studies were Asian. The treatment sequence of the included studies was simply described as EGFR-TKIs + brain RT and EGFR-TKIs alone.

Full table
TKI + brain RT vs. TKI alone on OS and iPFS
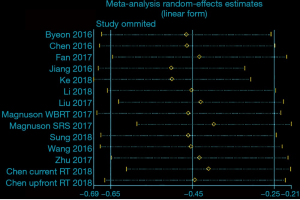
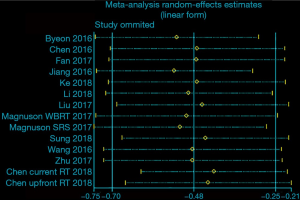
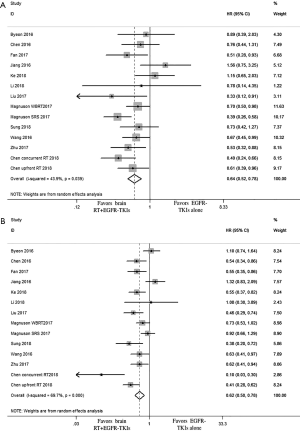
A total of 12 articles focusing on the comparison of clinical outcomes between EGFR-TKIs plus brain RT and EGFR-TKIs alone were included. EGFR-TKIs plus brain RT was demonstrated a statistically significant improvement in OS (HR =0.64, 95% CI: 0.52–0.78; P<0.001) (Figure 2A) and iPFS (HR=0.62, 95% CI: 0.50–0.78; P<0.001) (Figure 2B) and the pooled analyses display moderate heterogeneity in OS (P=0.039, I2 =43.9%) and significant heterogeneity in iPFS (P<0.001, I2 =69.7%). Then we conducted the influence analysis of the included data (Figures S1,S2), and founded out that Jiang et al., Ke et al. and Magnuson (SRS) et al. were the main origins which influenced the pooling outcome (26,29,32). The heterogeneity was effectively decreased or removed after exclusion of these three studies (I2 =0.0%, P=0.680); Moreover, I2 of iPFS was decreased to 38.5% (P=0.107) after removal of the three studies (byeon et al., Jiang et al. and Chen et al.) (23,26,27) which were considered as the culprit of heterogeneity by sensitivity analysis. The Egger’s and Begg’s test of included studies suggested no significant publication bias (P>0.05).
Meta-regression
To further explore the source of heterogeneity of iPFS and OS results, we did the meta-regression analyses with respect to age, gender, proportion of ECOG performance score, smoking status, mutation status, proportion of number of brain metastases, proportion of asymptomatic patients, proportion of extracranial metastases. For iPFS, the meta regression analysis demonstrated that the proportion of ECOG performance score (2+ vs. 0-1, P=0.070) and the proportion of brain symptomatic patients (no vs. yes, P=0.077) were potential factors that contributed to the heterogeneity; for OS, the ratio of younger vs. older patients was inclined to related to heterogeneity (P=0.075).
Dominant subgroup analyses
We further conducted a dominant subgroup analysis based on the median proportion of baseline characteristics of patients in the included studies (Table S1). In terms of iPFS, the dominant subgroup analysis suggested that symptomatic patients achieved a significant prolonged iPFS from combined therapy compared to asymptomatic patients (P<0.001, I2=93.7%) (Figure S3A). Moreover, patients with younger age (P=0.20) and the extracerebral metastases status (P=0.20) seems to benefit more from combined therapy compared to patients with old age and no extra metastasis. Nevertheless, contrary to the results of regression analysis, we found that the efficacy of combined therapy on iPFS was similar whatever the PS score patients achieved (P=0.79); In addition, gender (P=0.73), smoking status (P=0.72), the number of brain metastases (P=0.48), as well as EGFR mutation subtype (P=0.93) had no influence on iPFS for patients receiving the combination therapy (Figure S3B).
Full table
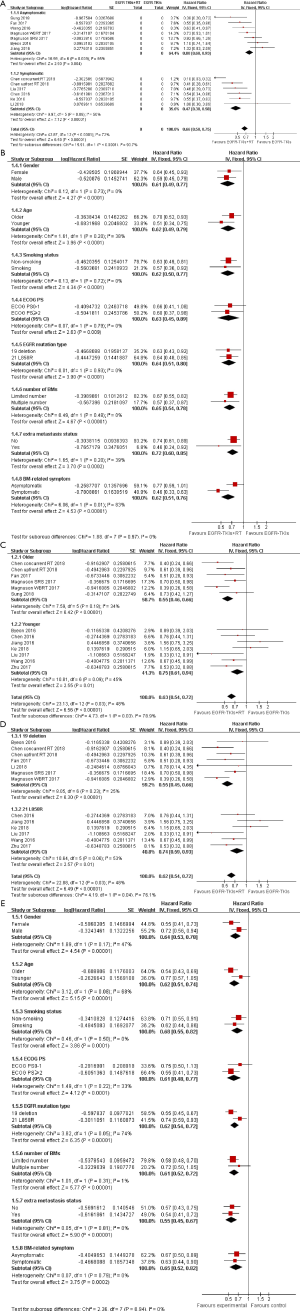
As for OS, the impact of combined therapy on OS was different according to age and mutation type. Patients with older age (P=0.03) and 19deletion (P=0.04) benefited more from the combination therapy of EGFR-TKIs and brain RT, compared with younger patients and patients with L858R (Figure S3C and Figure S3D). In addition, patients who termed female (P=0.17) or PS >2 (P=0.22) were potentially more likely to benefit from combined therapy. However, our results manifested that smoking status (P=0.50), BM-related symptom (P=0.79), the number of BMs (P=0.31) and extracranial metastasis(P=0.81) were not affect OS for patients receiving combined therapy (Figure S3E).
Direct subgroup analyses
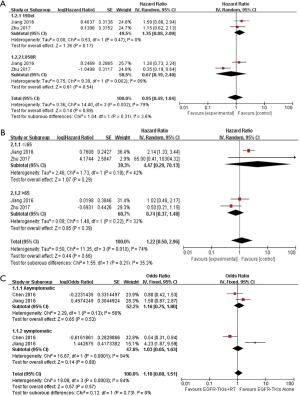
We also conducted subgroup analysis about iPFS with available information in several aspects: EGFR mutation subtype (mainly exon 21 L858R and exon 19del), age (>65 years old and ≤65 years old), sex (male and female), number of brain metastasis (≤3 vs. >3). There were only two studies [Zhu (24) and Jiang (26)] available for subgroup analyze. The results suggested that 21 L858R (HR =0.67, 95% CI: 0.19–2.40) intend to favor combined therapy while 19deletion (HR =1.35, 95% CI: 0.88–2.09) incline to another direction. Nevertheless, P-value of subgroup difference was 0.31 (Figure 3A). In addition, patients>65 years old (HR =0.74, 95% CI: 0.37–1.48) seemed to benefit more from combination of EGFR-TKI plus brain RT compared with patients ≤65 years old (HR =4.47, 95% CI: 4.47–70.13) (Figure 3B), but the subgroup difference also remained insignificant (P=0.21). Though our results suggested that asymptomatic patients (HR =1.16, 95% CI: 0.75–1.80) tend to favor EGFR-TKIs alone compared to patients with BM-related symptom, the difference between groups was not statistically significant (P=0.73) (Figure 3C), As for gender, HRs of female and male patients were similar.
Discussion
This study was mainly focused on the comparison of clinical efficacy between EGFR-TKIs plus brain RT and EGFR-TKIs alone in EGFR mutant NSCLC patients with BM. And furtherly intend to figure out advantage subgroup which benefit more from combined therapy. The study included 12 studies enrolling 1,553 NSCLC patients harboring EGFR mutation and BM. This pooled analysis demonstrated a significant difference in terms of OS (HR =0.64, 95% CI: 0.52–0.78; P<0.001) and iPFS (HR =0.62, 95% CI: 0.50–0.78; P<0.001) between combined therapy group and EGFR-TKIs alone group, indicating that the combined therapy may be a favorable option for the first-line treatment of these patients. Notably, the current comparisons were not based on randomization clinical trials. In addition, the adverse events (AEs) could not be assessed. Therefore, whether we should choose combination EGFR-TKI and radiotherapy over EGFR-TKI alone remained inconclusive.
BM was reported frequently occurred in EGFR mutated NSCLC, with approximately 8–49% happened at the initial diagnosis and about 24% during treatment course (35,36). An Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) demonstrated that factors including patients age, Karnofsky Performance Status (KPS), extracranial metastases (ECM), number of BMs and gene status (EGFR and ALK) were prognostic index for NSCLC patients with BM (37). However, the DS-GPA cannot be used to assess the effect of diverse treatment due to its inherent selection bias. Since the pooled analysis has suggested that the combination therapy is superior to single therapy, we furtherly seek to figure out the advantage groups by subgroup analysis.
For iPFS, our subgroup results showed that for EGFR mutated BM patients with combined therapy of brain RT and EGFR-TKI, symptomatic patients (HR =0.47, 95% CI: 0.38–0.58) apparently have a longer iPFS compared to asymptomatic patients (HR =0.80, 95% CI: 0.68–0.93) (P<0.0001). To our knowledge, it was valid optimal for asymptomatic patients with EGFR mutation and BM to choose EGFR-TKIs, and patients with BM-related symptom are more inclined to received brain RT plus EGFR-TKIs compared to EGFR-TKIs alone, these may interference the results. We also found that exon 21 L858R mutation (HR =0.67, 95% CI: 0.19–2.40) might benefited more from EGFR-TKIs + RT, though there was no statistical difference between two groups (P=0.31). The possible reasons were as follows. Firstly, from the aspect of clinical characteristics between mutation subtypes, a retrospective study on 1,063 patients has demonstrated that exon 19 deletion rather than exon 21 mutation was associated with high incidence of developing BM during the course of therapy (38), which complied with the results founded in Li et al. in 2015 (39). Another study further demonstrated that BM lesions with L858R mutation were located significantly closer to the brain surface (including preferential involvement of the caudate, cerebellum, and temporal lobe) than lesions with exon 19 deleted or wild-type EGFR (40). Both clinical features would have an impact on the treatment outcome. Secondly, the drug concentration affecting iPFS differed between these two mutations. Okuda et al. found that the plasma concentration of gefitinib was associated with the difference in PFS between subtypes of EGFR mutation, and showed that for the patients with exon 19 deletions, there was no significant difference in PFS between the high and low plasma concentration groups (median survival: 12.0 vs. 17.0 months, P=0.9548), In contrast, the iPFS of 21 L858R mutated patients was significantly different between low and high concentrations of gefitinib (median survival: 8.0 vs. 16.0 months, P<0.05) (41), which indicated that iPFS in patients with exon 21 mutation relied more on concentration than 19deletion patients; and since combined WBRT was associated with elevated CSF concentration of EGFR-TKIs by breaking BBB, both of them seem to partly explain why exon 21 mutation favors combined therapy. Moreover, several previous studies demonstrated that exon 19 and 21 mutations had different response to gefitinib or erlotinib, and patients with exon 19 deletion had longer progression-free survival than those with exon 21 L858R mutation when treated with EGFR-TKIs (42-44). This may explain the difference of additional value of brain RT as a complement to EGFR-TKIs. In addition, our results also suggested that patients aged more than 65 years old (HR =0.74, 95% CI: 0.37–1.48) achieved a potential better iPFS after combined therapy compared to patients aged less than 65 years old, while no statistically difference was observed (P=0.21). Moreover, male patients who received combined therapy were inclined to have a longer iPFS than woman (HR =0.59, 95% CI: 0.50–0.70, P=0.09). Kim et al. has demonstrated that the young cancer patients were characterized by more female, non-smokers and higher rate of distant metastasis compared with the older patients (45). It was also confirmed that younger cancer patients have a higher percentage of exon 19 deletion than L858R (45). Thus, it was rational that older patients and male may have a better iPFS after combined therapy. However, there still high heterogeneity existed in this analysis. The discrepancy may due to the limited number patients less than 65 years old enrolling in the retrospective studies.
As for OS, this study showed that the prognosis of older patients after the combined therapy were significantly better than that of younger patients. It was discordant with the findings that age >65 years old was a poorer prognosis factor for EGFR mutated NSCLC patients with BM following by WBRT and EGFR-TKI therapy (46). We supposed that may due to clinically more younger patients are tend to receive EGFR-TKIs alone because of the intolerance of potential side-effect of brain RT on the nervous system. Moreover, we found that patients with 19deletion achieved a significant improved OS than patients with 21 L858R after the combination therapy, which was consistence with previous study that 19deletion was a favorable prognosis factor for NSCLC patients with BM (44).
Thus, we suggested that mutation subtype, patient age, and BM-related symptom should be considered when determining treatment option in EGFR positive NSCLC patients with BM and be considered as a crucial stratification factor when designing future studies.
Previous studies mainly concentrated on the safety of EGFR-TKIs plus radiotherapy in EGFR mutated patients with BM (47). There were limited investigations focusing on the comparison of clinical efficacy between EGFR-TKIs plus radiotherapy and EGFR-TKIs; and most of the existing studies were conducted on patients without clear EGFR mutation status, which may affect the reliability of results. Our meta-analysis had several advantages. Firstly, all enrolled studies were conducted on the targeted population which are NSCLC patients harboring EGFR mutation and BM at the first diagnosis. Secondly, all of the included studies were comparative studies and the treatments were given as first-line therapy. Thus, the pooled results have certain value in guiding clinical treatment.
There were several limitations in this meta-analysis. Firstly, the number of included studies were relatively small and most of them were retrospective studies. Secondly, the insufficient of subgroup analysis data between two treatment groups abrogated further selection of dominant subgroups. Thirdly, patients involved in our meta-analysis were almost all Asians, hence further investigations on Caucasians and other races are required. Finally, the treatment toxicity, an important factor in choosing treatment options, was unavailable in this study.
Conclusions
In the first-line management of NSCLC patients with EGFR mutation and BM at the first diagnosis, the combination therapy presents significant improvement in OS and iPFS. Patients with BM-related symptoms, older age and 19deletion might benefit more from combined therapy. However, more randomized clinical trials and additional fundamental researches are still needed to further clarify the beneficial population of different therapy and its possible mechanism, so as to better guide clinical treatment.
Acknowledgments
Funding: Chinese National Natural Science Foundation (Grant No. 81501996); Key Project of Guangzhou Scientific Research Project (Grant No. 201804020030); Guangdong Doctoral Launching Program (Grant No. 2014A030310460); and Doctoral Launching Program of Guangzhou Medical University (Grant No.2014C27).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Luo D, Ye X, Hu Z, et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol 2014;35:2437-44. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Mujoomdar A, Austin JHM, Malhotra R, et al. Clinical Predictors of Metastatic Disease to the Brain from Non–Small Cell Lung Carcinoma: Primary Tumor Size, Cell Type, and Lymph Node Metastases1. Radiology 2007;242:882-8. [Crossref] [PubMed]
- Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 2014;40:716-22. [Crossref] [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii56-64. [Crossref] [PubMed]
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 2003;21:2529-36. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Bhatt VR, Kedia S, Kessinger A, Ganti AK. Brain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol 2013;31:3162-4. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707-16. [Crossref] [PubMed]
- Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99:283-6. [Crossref] [PubMed]
- Berger LA, Riesenberg H, Bokemeyer C, et al. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer 2013;80:242-8. [Crossref] [PubMed]
- Johung KL, Yao X, Li F, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res 2013;19:5523-32. [Crossref] [PubMed]
- Das AK, Sato M, Story MD, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res 2006;66:9601-8. [Crossref] [PubMed]
- Gow CH, Chien CR, Chang YL, et al. Radiotherapy in Lung Adenocarcinoma with Brain Metastases: Effects of Activating Epidermal Growth Factor Receptor Mutations on Clinical Response. Clin Cancer Res 2008;14:162-8. [Crossref] [PubMed]
- Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget. 2015;6:8366-76. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Fan Y, Huang Z, Fang L, et al. A phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol 2015;76:517-23. [Crossref] [PubMed]
- Jiang T, Min W, Li Y, et al. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med 2016;5:1055-65. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Byeon S, Ham JS, Sun JM, et al. Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non-small cell lung cancer and brain metastasis. Med Oncol 2016;33:97. [Crossref] [PubMed]
- Zhu Q, Sun Y, Cui Y, et al. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC). Oncotarget. 2017;8:13304-11. [PubMed]
- Chen Y, Yang J, Li X, et al. First-line epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitor alone or with whole‐brain radiotherapy for brain metastases in patients with EGFR‐mutated lung adenocarcinoma. Cancer Sci 2016;107:1800-5. [Crossref] [PubMed]
- Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT Demonstrated No Survival Benefit Other Than That of TKIs Alone in Patients with NSCLC and EGFR Mutation and Brain Metastases. J Thorac Oncol 2016;11:1718-28. [Crossref] [PubMed]
- Chen H, Wu A, Tao H, et al. Concurrent versus sequential whole brain radiotherapy and TKI in EGFR-mutated NSCLC patients with brain metastasis: A single institution retrospective analysis. Medicine 2018;97:e13014. [Crossref] [PubMed]
- Fan Y, Xu Y, Gong L, et al. Effects of icotinib with and without radiation therapy on patients with EGFR mutant non-small cell lung cancer and brain metastases. Sci Rep 2017;7:45193. [Crossref] [PubMed]
- Ke SB, Qiu H, Chen JM, et al. Therapeutic Effect of First-line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR-TKI) Combined with Whole Brain Radiotherapy on Patients with EGFR Mutation-positive Lung Adenocarcinoma and Brain Metastases. Curr Med Sci 2018;38:1062-8. [Crossref] [PubMed]
- Li SH, Liu CY, Hsu PC, et al. Response to afatinib in treatment-naïve patients with advanced mutant epidermal growth factor receptor lung adenocarcinoma with brain metastases. Expert Rev Anticancer Ther 2018;18:81-9. [Crossref] [PubMed]
- Liu Y, Deng L, Zhou X, et al. Concurrent brain radiotherapy and EGFR-TKI may improve intracranial metastases control in non-small cell lung cancer and have survival benefit in patients with low DS-GPA score. Oncotarget 2017;8:111309-17. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Abraham J, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor–Naive Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Sung S, Lee SW, Kwak YK, et al. Intracranial control and survival outcome of tyrosine kinase inhibitor (TKI) alone versus TKI plus radiotherapy for brain metastasis of epidermal growth factor receptor-mutant non-small cell lung cancer. J Neurooncol 2018;139:205-13. [Crossref] [PubMed]
- Wang W, Song Z, Zhang Y, et al. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell lung cancer patients who develop brain metastasis. Arch Med Sci 2018;14:1298-307. [Crossref] [PubMed]
- Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer 2014;84:86-91. [Crossref] [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in non-small cell lung cancer. Neuro Oncol 2010;12:1193-9. [Crossref] [PubMed]
- Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. [Crossref] [PubMed]
- Li H, Cao J, Zhang X, et al. Correlation between status of epidermal growth factor receptor mutation and distant metastases of lung adenocarcinoma upon initial diagnosis based on 1063 patients in China. Clin Exp Metastasis 2017;34:63-71. [Crossref] [PubMed]
- Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol 2015;124:79-85. [Crossref] [PubMed]
- Takano K, Kinoshita M, Takagaki M, et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol 2016;18:716-24. [Crossref] [PubMed]
- Okuda Y, Sato K, Sudo K, et al. Low plasma concentration of gefitinib in patients with EGFR exon 21 L858R point mutations shortens progression-free survival. Cancer Chemother Pharmacol 2017;79:1013-20. [Crossref] [PubMed]
- Sekine A, Satoh H, Iwasawa T, et al. Prognostic factors for brain metastases from non-small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol 2014;31:228. [Crossref] [PubMed]
- Won YW, Han JY, Lee GK, et al. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol 2011;64:947-52. [Crossref] [PubMed]
- Li H, Zhang X, Cao J, et al. Exon 19 deletion of epidermal growth factor receptor is associated with prolonged survival in brain metastases from non-small-cell lung cancer. Tumour Biol 2015;36:7333-4. [Crossref] [PubMed]
- Kim L, Ho KK, Han YY, et al. Clinicopathologic and Molecular Characteristics of Lung Adenocarcinoma Arising in Young Patients. J Korean Med Sci 2012;27:1027-36. [Crossref] [PubMed]
- Wei H, Su M, Lin R, et al. Prognostic factors analysis in EGFR mutation-positive non-small cell lung cancer with brain metastases treated with whole brain-radiotherapy and EGFR-tyrosine kinase inhibitors. Oncol Lett 2016;11:2249-54. [Crossref] [PubMed]
- Zhang Y, He D, Fang W, et al. The Difference of Clinical Characteristics Between Patients With Exon 19 Deletion and Those With L858R Mutation in Non-small Cell Lung Cancer. Medicine 2015;94:e1949. [Crossref] [PubMed]