Influence and mechanism of lung cavitation development on antiangiogenic therapy
Introduction
Lung cavitation often occurs in lung cancer patients who have failed multiple lines of chemotherapy and have been treated with antiangiogenic agents (1,2). It is often considered a risk factor for the deterioration of a patient’s survival status (3). However, results may vary depending on the drug used. In a previous phase II trial, which included 67 non-small cell lung cancer (NSCLC) patients treated with bevacizumab, lung cavitation was associated with patient death (3). In another study of cediranib, Crabb et al. reported no clear link between clinical response and tumor cavitation in NSCLC patients (4). It is essential to study the relationship between lung cavitation development and antiangiogenic therapy to avoid serious adverse events and guide clinical treatments. Apatinib, a novel small-molecule vascular endothelial growth factor receptor (VEGFR) inhibitor, has been approved to treat advanced or metastatic chemorefractory gastric cancer in China (5,6). Recently, apatinib has been shown to have positive effects on multiple tumors, including lung cancer (6,7). According to our observations, apatinib-treated patients with lung lesions usually develop lung cavitation although the clinical significance of this finding has not been studied yet. Herein, we retrospectively reviewed apatinib-treated patients to evaluate the clinical significance of lung cavitation development during apatinib therapy (Clinical Trial Register No. NCT03629691). This is the first study concerning lung lesions of both primary and metastatic lung cancer. The study design was accepted as a poster at the 18th World Conference on Lung Cancer (WCLC, October 15–18, 2017, Yokohama, Japan P3.03-014).
Methods
Clinical study
Patients
Between February 1, 2015, and May 19, 2018, 433 adult patients with gastric adenocarcinoma, lung adenocarcinoma, and lung squamous cell carcinoma, who had failed multiple lines of chemotherapy and lacked a standard treatment regimen, received 250 mg oral apatinib daily at the Affiliated Hospital of Qingdao University. All patients had an Eastern Cooperative Oncology Group performance status of 0 to 1, with stable hepatic, hematological, and renal functions. Patients with cardiac disease or hemorrhagic disease were excluded. Other patient demographic and clinical data, including histopathology, age, gender, disease stage, apatinib-administration inclusion and withdrawal dates, adverse events, oncologic clinical response, comorbid conditions, and smoking history, were retrieved from the Current Research Information System (CRIS) database. The study was approved by the institutional review board of the Affiliated Hospital of Qingdao University (No. QYFYEC 2015-006-07) and was registered as a clinical trial (No. NCT03629691).
Evaluation of clinical responses
All patients underwent a pretherapy chest computed tomography (CT) examination before apatinib treatment, which was performed an average of 7.3 days before initiating therapy, followed by chest CT examination at least once every four weeks. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were the duration of locoregional control (LRC), overall survival (OS), quality of life, and safety. For metastatic lung cancer, LRC indicates local control of the metastatic lung tumors. Evaluation of the oncologic clinical responses was based on the alternate modified method published by Crabb et al. (4). This method was used to target lesions in which the longest diameter of any cavitation (zero if no cavity was present) was subtracted from the longest total lesion diameter, with each measurement taken in the same plane, to provide an alternate method to calculate the sum of the measurements for all target lesions.
Statistical methodology
The association between cavitation formation and the apatinib treatment effect was investigated. Number of patients, number of events, median time-to-event endpoints (LRC, OS, and PFS) and corresponding 95% confidence intervals (CIs) were evaluated for each subgroup; 95% CIs were calculated per Brookmeyer and Crowley method, and hazard ratios (HRs, including 95% CIs) were calculated using the Cox proportional hazards model. The Cox proportional hazards model was fitted to the data from all cavitation patients, with cavitation and biomarker statuses, as well as their interactions, as explanatory variables. The interactions between the treatment effect and biomarkers were tested using a two-sided Wald’s test.
Zebrafish experiments
Zebrafish care and handing
Apatinib (purity >98%) was purchased from MERYER (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO) to obtain stock concentrations of 5 mM and 500 µM. Fetal bovine serum (FBS), phosphate-buffered saline (PBS), Roswell Park Memorial Institute basal medium 1640 (RPMI 1640 medium), penicillin, and streptomycin were purchased from Basal Media Technologies (Shanghai, China). H1299 and SCG-7901 cell lines were obtained from the American Type Culture Collection (ATCC) and fluorescently labeled with CM-DiI (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cell viability was assessed by trypan blue staining before injection. The time length is indicated as days post-apatinib treatment (dpt). Transgenic zebrafish Tg (fli-1: EGFP) expressing enhanced green fluorescent protein (EGFP) in the endothelial cells were obtained from the Model Animal Research Center of Nanjing University. All zebrafish assays in our study were conducted before 120 hours post fertilization (hpf), as events before this time point are considered to be non-protected life stages in the European Union and are therefore accepted as an alternative to animal testing (8). Embryos and larvae were staged as described by Kimmel et al. (9). The labeled cells were injected into the yolk sac of the zebrafish embryo using stereoscopic microinjection (SMZ 745 T; Nikon, Japan).
Angiogenesis inhibition and cell proliferation suppression
Xenografted embryos were treated with 0.1, 0.5, and 0.25 µM apatinib by soaking and incubating at 32 °C to observe angiogenesis inhibition and cell proliferation suppression. We monitored angiogenesis and tumor cell growth in vivo at 1, 2, and 3 dpt using an inverted fluorescence microscope (IX71; Olympus, Japan). Angiogenesis was observed using a confocal microscope (LSM710; ZEISS, Germany). The studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the Affiliated Hospital of Qingdao University.
Statistical methodology
All statistical results were expressed as the mean ± SEM and were generated using GraphPad Prism 5.0. The decreases/increases in the fold of change were analyzed using one-way ANOVA, followed by Dunnett’s multiple comparison test. Significance was considered when P value was less than 0.005. *** indicates statistical significance P<0.005, ** indicates P<0.01, and * indicates P<0.05. All experiments were performed in triplicate, and the independent experiment was repeated at least three times.
Anticancer mechanism analysis
Cell culture
H1299 and SCG-7901 cell lines were routinely cultured in 175-mm flasks in serum-containing DMEM. Cells were placed in a modular incubator chamber (Billups Rothenberg, Inc., Del Mar, CA, USA) and flushed for 10 minutes with a gas mixture of 5% CO2-95% N2 to achieve and control hypoxic conditions; the final medium pO2 value was consistently below the 0.5% to 1% range. The chamber was then sealed and placed at 37 °C in a conventional cell incubator.
Western-blot analysis
Protein expression in cultured cells was analyzed by western blotting, which was performed with equal amounts of whole-cell extracts obtained with RIPA buffer (Beyotime, China). Cells were washed with ice-cold phosphate-buffered saline (PBS) and collected by scraping. Protein extraction from cells was carried out. Protein levels in the extracts were quantified using the BCA protein assay (Beyotime, China). Western blots were generated and probed with specific primary antibodies against VEGFR-2, HIF-α, Cyclin D1, and P53. All antibodies were obtained from Abcam (SA). Blots were developed using the enhanced chemiluminescence detection system.
Results
Effect of lung cavitation development on survival
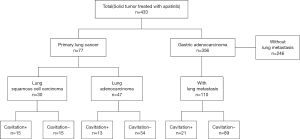
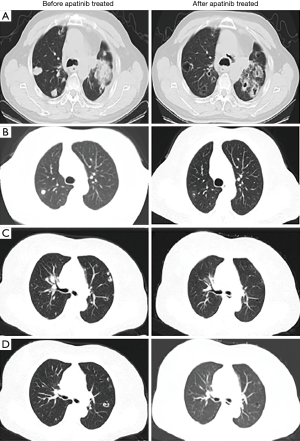
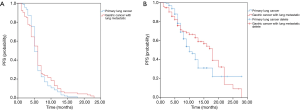
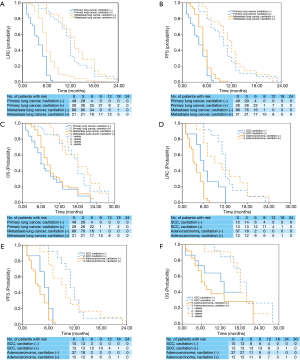
In total, 187 patients with pulmonary tumor lesions were included in our study. Seventy-seven patients had lung cancer, and 110 had gastric adenocarcinoma (Figure 1). Table 1 shows the baseline characteristics of primary and metastatic lung cancer patients. The median age of the study subjects was 58.2 years (range, 33–79 years). Age (P=0.35), sex (P=0.23), tumor stage (P=0.06), pneumonia status (P=0.59) and adverse event occurrences (P=0.76, 0.48, and 0.14 for hypertension, skin rash, and proteinuria, respectively) did not differ between the primary and metastatic lung cancer cohorts. More primary lung cancer patients than metastatic lung cancer patients underwent pulmonary surgery (P=0.002) or radiotherapy (P<0.001). Figure 2 shows cases of lung cavitation development during apatinib therapy. Survival analysis indicates that lung cavitation development improved LRC, PFS, and OS, regardless of whether patients had primary or metastatic lung cancer (Figures 3,4). According to Table 2, in patients without lung cavitation, gastric cancer patients benefited more from apatinib therapy than did primary lung cancer patients. However, when lung cavitation developed, the selectivity of apatinib became weak. In the primary lung cancer cohort, histology did not influence the effect of apatinib therapy (Table 3).

Full table

Full table

Full table
Anticancer mechanism of apatinib
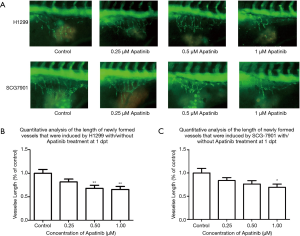
Angiogenesis inhibition
Figure 5A shows vessel growth inhibition properties of apatinib in different tumor types. As expected, apatinib inhibited vessel growth induced by both cell lines early at 1 dpt. When the dose was increased from 0.25 to 1 µM, apatinib decreased the development of H1299-cell-induced intact subintestinal vessels (SIVs) in zebrafish from a basket to several branches. However, for SCG-7901-cell-induced SIVs, the changes were not significant. Vessel length of control zebrafish was set as the baseline and was normalized to 1. At 1 dpt the H1299-cell-induced SIV lengths were reduced to 0.82, 0.68, and 0.66 fold in the 0.25, 0.5, and 1 µM apatinib treatment groups respectively (Figure 5B). In contrast, SCG-7901-cell-induced SIV lengths reduced to 0.84, 0.76, and 0.69 fold in the 0.25, 0.5, and 1 µM apatinib treatment groups respectively (Figure 5C). Taken together, these results indicate that the vascular proliferation induced by H1299 cell lines showed higher sensitivity to apatinib than that caused by the SCG-7901 cell lines.
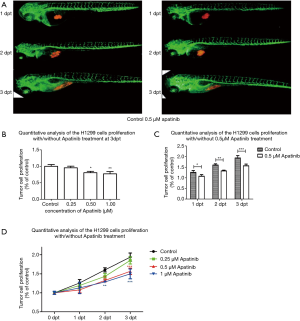
Cell proliferation suppression
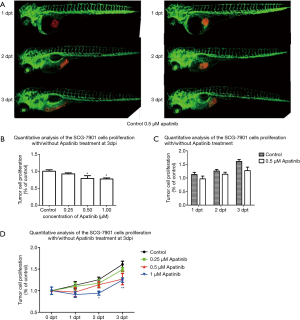
H1299 and SCG-7901 cells were xenografted into zebrafish soaked in apatinib 0.25–1 µM at 1–3 dpt. The cell number at 0 dpt was set as the baseline and normalized to 1. At 3 dpt, the H1299 cells proliferated by 1.93 fold in the control group, and 1.85, 1.56 and 1.50 fold in the 0.25, 0.5, and 1 µM apatinib treatment groups, respectively (Figure 6). SCG-7901 cells exhibited a 1.61-fold increase in the control group at 3 dpt, but were 1.49, 1.27, and 1.25 fold in the 0.25, 0.5 and, 1 µM apatinib treatment groups, respectively (Figure 7). These results indicate the weak selectivity of apatinib in cell proliferation inhibition in vivo.
Molecular mechanism analysis
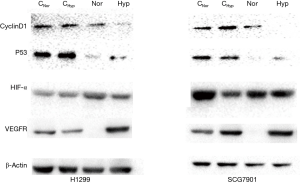
Protein expression of VEGFR, HIF-α, Cyclin D1, and P53 in control and 0.5 µM apatinib treatment groups of H1299 and SCG-7901 cell lines in normoxic and hypoxic environments was evaluated. The results are described in Figure 8. In normoxia, apatinib decreased the protein expression of VEGFR and P53 but did not affect the expression of Cyclin D1 or HIF-α in both cell lines. In hypoxia, the protein expression of Cyclin D1 and P53 in both cell lines treated with apatinib was significantly lower than the control. However, the protein expression of VEGFR was not decreased. Furthermore, the expression of HIF-α was increased.
Discussion
Lung cavitation development is a potential prognostic biomarker of apatinib therapy
Apatinib is the first small-molecule VEGFR inhibitor approved to treat advanced or metastatic chemorefractory gastric cancer in China (10). Additionally, it has been confirmed to increase the duration of LRC and survival in patients with multiple solid tumors (5-7,11-21). As we have observed, many patients treated with apatinib develop cavitation in their lung lesions. Previously, several clinical trials evaluated the effect of the cavitation status on the efficacy of antiangiogenic drug treatment in patients with NSCLC (1-4). However, the relationship between tumor cavitation development and clinical response remains uncertain, while studies exploring the potential lung cavitation development induced by apatinib treatment are lacking. To explore the clinical significance of this phenomenon during apatinib therapy, we evaluated the predictive roles of lung cavitation development during apatinib treatment in both primary and metastatic lung cancers. This is the first study of lung cavitation development in metastatic tumors. According to our results, regardless of whether patients had primary or metastatic lung cancer, lung cavitation development was associated with better efficacy of apatinib, and may be a potential prognostic biomarker in apatinib therapy.
Apatinib inhibited tumor growth by both vascular proliferation suppression and cell proliferation inhibition
Among patients without lung cavitation development, primary lung cancer patients benefited more from apatinib therapy than metastatic lung cancer patients. However, in patients with lung cavitation, tumor types did not influence the clinical response to apatinib treatment. In the vascular proliferation suppression experiments with zebrafish, SIVs induced by lung tumor cells were more sensitive to apatinib than those caused by gastric tumor cells, which followed the clinical outcomes of the patients without lung cavitation development. Also, cell proliferation inhibition in vivo showed weak tumor type selectivity for apatinib, which was consistent with the clinical results of the patients with lung cavitation development. As the zebrafish experiments showed, apatinib inhibited tumor growth by both vascular proliferation suppression and cell proliferation inhibition. Interestingly, in a hypoxic environment, apatinib increased the expression of HIF-1α and did not affect VEGFR expression, which was quite different from the effects observed in a normoxic environment. According to these results, we deem it necessary to further study the anticancer mechanism of apatinib.
Limitation
This study had several limitations. This was a retrospective analysis of the cavitation status in a previously unselected population, and the patient numbers in some groups were small. Therefore, some differences in tumor baseline characteristics could not be controlled. In vivo experiments were performed only in zebrafish; more cellular trials and mouse studies should be designed for further research. Because better outcomes are expected in patients with lung cavitation, it may take years before enough progression events or deaths occur to facilitate assessing efficacy in this population. When available, the final dataset, including the effectiveness and late toxicity endpoints, will provide valuable insight into differences in survival and quality of life between patients who develop cavitation and those who do not.
Conclusions
In conclusion, the analysis of this trial showed that cavitation development was beneficial in apatinib-treated patients and functioned as a prognostic biomarker.
Acknowledgments
Funding: This work was supported by the Taishan Scholar Foundation (grant tshw 201502061 to X Zhang), Qingdao People’s Livelihood Science and Technology Program (grant 16-6-2-3-nsh to X Zhang and 18-2-2-74-jch to M Jiang), Chinese Postdoctoral Science Foundation (2017M6122218 to M Jiang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of the Affiliated Hospital of Qingdao University (No. QYFYEC 2015-006-07) and written informed consent was obtained from all patients.
References
- Onn A, Choe DH, Herbst RS, et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology 2005;237:342. [Crossref] [PubMed]
- Marom EM, Martinez CH, Truong MT, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol 2008;3:351-7. [Crossref] [PubMed]
- Huang C, Wang X, Wang J, et al. Incidence and clinical implication of tumor cavitation in patients with advanced non-small cell lung cancer induced by Endostar, an angiogenesis inhibitor. Thoracic Cancer 2014;5:438-46. [Crossref] [PubMed]
- Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor Cavitation: Impact on Objective Response Evaluation in Trials of Angiogenesis Inhibitors in Non-Small-Cell Lung Cancer. J Clin Oncol 2009;27:404-10. [Crossref] [PubMed]
- Fornaro L, Vasile E, Falcone A. Apatinib in Advanced Gastric Cancer: A Doubtful Step Forward. J Clin Oncol 2016;34:3822-3. [Crossref] [PubMed]
- Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer 2017;84:184-92. [Crossref] [PubMed]
- Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc) 2015;51:223. [Crossref] [PubMed]
- Strähle U, Scholz S, Geisler R, et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 2012;33:128-32. [Crossref] [PubMed]
- Kimmel CB, Ballard WW, Kimmel SR, et al. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253-310. [Crossref] [PubMed]
- Huang PW, Chou W, Shen W, et al. Hand-foot skin reaction predicts treatment outcome of pazopanib in patients with metastatic soft tissue sarcoma: A multicenter study in the Asian population. Asia Pac J Clin Oncol 2018;14:353-60. [Crossref] [PubMed]
- Brower V. Apatinib in treatment of refractory gastric cancer. Lancet Oncol 2016;17:e137. [Crossref] [PubMed]
- Dou S, Zhang L, Li R, et al. Phase 2 Study of Apatinib, A Novel VEGFR Inhibitor in Patients With Recurrent and/or Metastatic Adenoid Cystic Carcinoma of the Head and Neck: Preliminary Results. Int J Radiat Oncol Biol Phys 2018;100:1378-9. [Crossref]
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of Apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [PubMed]
- Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget 2017;8:29346-54. [PubMed]
- Kang Y, Ryu M, Park SR, et al. A phase II study of apatinib, a highly selective inhibitor of VEGFR-2, in patients with metastatic solid tumors without standard treatment options. Ann Oncol 2016;27:373. [Crossref]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Zhang M, Deng W, Cao X, et al. Concurrent apatinib and local radiation therapy for advanced gastric cancer: A case report and review of the literature. Medicine 2017;96:e6241. [Crossref] [PubMed]
- Li J, Xu N, Cheng Y, et al. A randomized, double-blind, multicenter, Phase 2, three-arm, placebo-control study of apatinib as third line treatment in patients with metastatic gastric carcinoma. Beijing: National Conference of clinical Oncology and 2012 CSCO Academic Conference, 2012.
- Shi Y, Sun Y, Yu J, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol 2017;13:87-103. [Crossref] [PubMed]
- Zhang H, Chen F, Wang Z, et al. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther 2017;10:837-45. [Crossref] [PubMed]
- Li F, Liao Z, Zhao J, et al. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget 2017;8:64471-80. [PubMed]