Rationale and design of a phase II study to evaluate prophylactic treatment of dacomitinib-induced dermatologic adverse events in epidermal growth factor receptor-mutated advanced non-small cell lung cancer (SPIRAL-Daco study)
Introduction
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are key drugs in patients with metastatic non-small cell lung cancer (NSCLC) harboring EGFR-activating mutations. Compared with first-generation EGFR-TKI (e.g., gefitinib, erlotinib), dacomitinib is the first second-generation EGFR-TKI to significantly improve overall survival (1). Progression-free survival was also significantly prolonged in dacomitinib treatment compared with gefitinib treatment [14.7 vs. 9.2 months; hazard ratio (HR) 0.50; 95% confidence interval (CI), 0.47–0.74], especially in the Asian population (18.2 vs. 10.9 months; HR 0.43; 95% CI, 0.32–0.59). However, the rate of any grade 3 adverse event (AE) increases in dacomitinib treatment compared with gefitinib treatment (51% vs. 30%) (2). The most common grade 3–4 AEs were dermatitis acneiform (14% vs. 0%). Skin and subcutaneous tissue disorders were the most frequent causes of permanent discontinuation due to dacomitinib-induced AEs (3).
Besides treatment interruption, dermatologic AEs can lead to dose reduction, as well as having a negative impact on patients’ quality of life and social functioning (4,5). The management strategy for dermatologic AEs is crucial in dacomitinib treatment. Topical antibiotics or topical corticosteroids are generally recommended for the initial treatment of mild cases (grade 1) of EGFR-TKI-related skin rash. For the more severe rash (≥ grade 2), oral antibiotics, mainly tetracycline class antibiotics, can be prescribed (6). Comprehensive pre-emptive treatment (skin moisturizer, sunscreen, topical steroid and minocycline) also significantly reduced drug-induced skin toxicities (≥ grade 2) compared with placebo among Japanese patients with metastatic colorectal cancer receiving panitumumab treatment (7).
The standard symptomatic treatment of EGFR-TKI-induced dermatologic AEs is topical corticosteroids; however, a proactive strategy has become desirable in clinical practice settings. Therefore, we conducted a phase II study to explore the preventive efficacy of prophylactic medication against dacomitinib-induced dermatologic AEs in patients with metastatic NSCLC.
Methods
Objective and endpoint
With the above background, the present study is underway to explore the efficacy of a prophylactic strategy for dermatologic AEs resulting from dacomitinib treatment in patients with advanced NSCLC harboring EGFR-activating mutations. The primary endpoint is the incidence of dermatologic AEs (≥ grade 2) during the first 8 weeks of dacomitinib treatment. The secondary endpoints are the incidence of dose reduction of dacomitinib during the first 8 weeks of dacomitinib treatment, safety, and progression-free survival.
Study design & settings
This is a single arm, prospective, open-label, multicenter, phase II trial.
Trial registration number: jRCTs071190015.
Eligibility criteria
Inclusion criteria
Patients with NSCLC (excluding squamous cell lung cancer):
- Confirmed histologically or cytologically;
- With clinical stage IIIB, IIIC, or IV disease or postoperative recurrence;
- Harboring EGFR-activating mutations;
- Naïve to dacomitinib treatment;
- Performance status (ECOG) of 0 to 2;
- Age ≥20 years;
- Having at least 1 measurable lesion according to RECIST criteria ver.1.1.
Patients with adequate organ function (bone marrow, liver, kidney, etc.) which fulfill the following criteria within 2 weeks prior to enrollment:
- White blood cell count ≥3,000/mm3, ≤12,000/mm3;
- Neutrophil count ≥1,500/mm3;
- Platelet count ≥100,000/mm3;
- Hemoglobin ≥9.0 g/dL;
- Aspartate aminotransferase ≤100 IU/L;
- Alanine aminotransferase ≤100 IU/L;
- Serum bilirubin ≤1.5 mg/dL;
- Serum creatinine ≤1.5 mg/dL;
- SpO2 (room air) ≥90%.
The following time is required between prior treatment and dacomitinib initiation.
- Chemotherapy: ≥2 weeks pass since the final administration of prior chemotherapy.
- EGFR-TKI: the next day after the last administration if there are dermatologic AEs of grade 1 or less.
- Radiation therapy: in the case of radical radiation for chest, ≥4 weeks have passed since the day of final radiation.
Patients who provide written informed consent are eligible to participate in the study.
Exclusion criteria
The participant must be excluded from the trial if the participant:
- Has a known history of prior malignancy except if the participant has undergone potentially curative therapy with no evidence of recurrence of that disease for 5 years since initiation of the curative therapy (the time requirement does not apply to participants who underwent successful definitive treatment for intramucosal carcinoma or carcinoma in situ);
- Is pregnant, breastfeeding, or expecting to conceive or father children within the projected duration of the study;
- Has a known psychiatric disorder which would make trial participation difficult;
- Has interstitial lung disease, drug-induced pneumonitis or radiation pneumonitis with active status;
- Has intestinal paresis or intestinal obstruction;
- Is unable to take oral medication;
- Has an active infection requiring intravenous antibiotics or anti-fungal therapy;
- Has spinal cord compression, or symptomatic and unstable brain metastases, except for patients who have completed definitive therapy, are not on steroids, and have a stable neurologic status for at least 2 weeks after completion of the definitive therapy and steroids;
- Has severe or uncontrolled systemic diseases, including uncontrolled hypertension, active bleeding diatheses, severe arrhythmia requiring medication, continuous diarrhea, unstable angina with symptoms within the past 3 weeks, or myocardial infarction within the past 6 months;
- Has a known sensitivity to any component of the following medications: minocycline, skin moisturizer containing heparinoid, topical steroid, and sunscreen;
- Is not suitable to participate in the trial, as judged by an investigator.
Dose and treatment regimens
Dacomitinib (45 mg orally, once daily) will be administered at a regular time. If patients forget to take the medication, it must be taken within 12 hours after the regular time, otherwise it should be skipped.
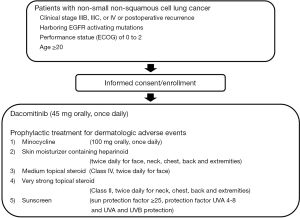
Prophylactic treatment for dermatologic AEs will start on day 1 and be administered through to 8 weeks after the initiation of dacomitinib. A schematic illustration of the study is provided in Figure 1. Pre-emptive treatment can continue after the study period as a choice and the details of the treatment are as follows:
- Minocycline (100 mg orally, once daily);
- Skin moisturizer containing heparinoid (twice daily for face, neck, chest, back, and extremities);
- Medium topical steroid (class IV, twice daily for face);
- Very strong topical steroid (class II, twice daily for neck, chest, back, and extremities);
- Sunscreen (sun protection factor ≥25, protection factor UVA 4–8 and UVA and UVB protection).
Withdrawal, discontinuation criteria
Patient cannot continue to receive the protocol treatment, if they meet the following criteria:
- Disease progression;
- Complications of interstitial lung disease;
- Complications of non-hematologic AEs (≥ grade 4);
- Three-fold dose reduction needed;
- Discontinuation of treatment with 3 weeks or more;
- Request for withdrawal from the study;
- Difficult to receive continuous treatment (due to relocation, hospital referral);
- Participant dies during the trial;
- Be worthy of exclusion from the trial for reasons that are clarified after enrollment;
- Investigator’s decision to discontinue the protocol treatment.
Prohibited concomitant treatments
The following treatments are prohibited during the ongoing trial:
- For the treatment of cancer: chemotherapy, immunotherapy, hormone therapy, biological response modifier treatment, radiation, surgery, or hyperthermia.
- Other investigational new drugs with an expected antineoplastic effect.
Patients cannot continue to receive the protocol treatment if these treatments are required during the trial period.
Rationale for setting the number of enrolled participants
From the data of ARCHER 1042 trial, the incidence of dermatologic AEs (≥ grade 2) in the first 8 weeks of dacomitinib treatment was reported to be 46% in the placebo group and 23% in the prophylactic doxycycline group (8). We determined an expected incidence proportion of 23% and a threshold incidence proportion of 46%. Assuming 90% power and a one-sided alpha level of 0.05, we calculated that we needed 36 subjects using a binominal test. Considering a 10% rate of loss to follow-up, we planned to enroll 40 subjects (9).
Population to be analyzed
We will use the full analysis set (FAS) for all analyses except for safety. We will also use the per-protocol set (PPS) for the primary analysis. For the safety analysis, we will provide the safety analysis set (SAS).
FAS: Whole enrolled trial population except as follows: (I) patients without informed consent; (II) patients who do not receive any protocol treatment; or (III) patient who retract their informed consent for this study. Final judgement will be made based on discussions among the principal investigator, the research center, the trial statistician, and the data center.
PPS: PPS includes FAS without the following: (I) violation of inclusion criteria; (II) violation of exclusion criteria; (III) violation of concomitant medication criteria; or (IV) violation of concomitant treatment criteria. Final judgement will be made based on discussion among the principal investigator, the research center, the trial statistician, and the data center.
SAS: SAS includes the whole enrolled trial population except as follows: (I) patients who do not receive any protocol treatment; or (II) patients who retract their informed consent for this study. Final judgement will be made based on discussion among the principal investigator, the research center, the trial statistician, and the data center.
Statistical methods
We will adopt a one-sided significance level of 0.05 for the primary end-point analysis, and a two-sided alpha level of 0.05 for the other analyses. We will not impute missing values. The principal investigator, the research center and the trial statistician will confer about clinical competence and data handling, including outlier values. We will estimate the incidence proportion and 90% confidence interval using the Wald method. The primary analysis will be met if the upper 90% confidence limit is 46% or less. For sensitivity analysis, we plan to do same analysis using the PPS. We will estimate the proportion of dose reduction of dacomitinib and 95% confidence interval using the Wald method. For progression-free survival, we will estimate the survival curve, median progression-free survival time, and annual survival rates using the Kaplan-Meier method. We will adopt the Brookmeyer and Crowley method for the estimation of median progression-free survival time and the Greenwood method for the estimation of the standard error of annual survival rates. For safety, we will use descriptive statistics and summarize the incidence and degree of AEs.
Discussion
Though dacomitinib is more effective than first-generation EGFR-TKIs, there are many situations in which side effects are considerable and management is difficult. Preventive treatments for skin disorders are commonly used for anti-EGFR antibody preparations that already have a strong association with similar skin disorders, which has contributed to improved treatment completion rates. Regarding dacomitinib, the clarification of the effects of prophylactic treatments on skin disorders may also establish more effective treatment methods. Study enrollment began in July 2019; accrual will take 1.5 years and the total study time is expected to be 3 years.
Acknowledgments
Funding: This project has received funding from Pfizer (sponsor) IIR grant agreement No. WI240974.
Footnote
Conflicts of Interest: J Uchino reports grants from Eli Lilly Japan K.K. that are outside of the submitted work. T Yamada reports grants from Nippon Boehringer Ingelheim and Ono Pharmaceutical Company that are outside of the submitted work. K Takayama reports grants from Chugai-Roche and Ono Pharmaceutical Company, personal fees from AstraZeneca K.K., Chugai-Roche, MSD-Merck, Eli Lilly, Boehringer-Ingelheim, and Daiichi-Sankyo that are outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The study protocol and informed consent documents were approved by the ethical committees of the participating institutions, Clinical Research Network Fukuoka Certified Review Board (No. 19-C01), and informed consent was obtained from all patients. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Mok TS, Cheng Y, Zhou X, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244-50. [Crossref] [PubMed]
- Passaro A, de Marinis F. Dacomitinib in EGFR-positive non-small cell lung cancer: an attractive but broken option. Transl Lung Cancer Res 2018;7:S100-S102. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Tischer B, Huber R, Kraemer M, et al. Dermatologic events from EGFR inhibitors: the issue of the missing patient voice. Support Care Cancer 2017;25:651-60. [Crossref] [PubMed]
- Hofheinz RD, Deplanque G, Komatsu Y, et al. Recommendations for the Prophylactic Management of Skin Reactions Induced by Epidermal Growth Factor Receptor Inhibitors in Patients With Solid Tumors. Oncologist 2016;21:1483-91. [Crossref] [PubMed]
- Goto A, Ozawa Y, Koda K, et al. Clinical impact of minocycline on afatinib-related rash in patients with non-small cell lung cancer harboring epidermal growth factor receptor mutations. Respir Investig 2018;56:179-83. [Crossref] [PubMed]
- Kobayashi Y, Komatsu Y, Yuki S, et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol 2015;11:617-27. [Crossref] [PubMed]
- Lacouture ME, Keefe DM, Sonis S, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol 2016;27:1712-8. [Crossref] [PubMed]
- Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: Chichester: Wiley, 1981.