
Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1). Around half of all lung cancer patients present several metastases at diagnosis (2), and non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases (3). Several types of molecularly targeted agents and immune checkpoint inhibitors (ICIs) have been developed for advanced lung cancer patients based on the advances in molecular biology, as evidenced by improved clinical outcomes, such as prolonged survival and more durable treatment response (4-8).
Ramucirumab (Ram) is a human recombinant IgG1 monoclonal antibody targeting the vascular endothelial growth factor (VEGF) receptor-2 (9). The REVEL study was a phase III clinical trial that compared the effectiveness of combination therapy of docetaxel (Doc) with Ram to that of Doc monotherapy in advanced NSCLC patients who showed disease progression after platinum-based chemotherapy. The results of this study demonstrated that the combination therapy significantly increased progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) compared to Doc monotherapy (10). Therefore, combination therapy with Doc plus Ram was considered as one of the standard treatments and has been approved in several countries, including the US and Japan, for the treatment of advanced or relapsed NSCLC patients with tumors that are refractory to platinum-based chemotherapy. However, only limited data is available regarding the therapeutic response to Doc plus Ram combination therapy in previously treated NSCLC patients.
ICIs are currently promising alternative treatments for NSCLC. Of them, nivolumab, pembrolizumab, and atezolizumab have been approved in the US, Japan, and other countries for the treatment of patients with metastatic NSCLC based on the findings of phase III trials, which showed superior outcomes of NSCLC patients treated with ICIs compared to patients treated with standard systemic chemotherapy (4-8,11). Based on indirect comparisons, a recent study reported that treatment with nivolumab and pembrolizumab significantly improved ORR compared to atezolizumab (12).
To identify the responders to ICI treatment, several mechanisms that mediate a favorable response to ICIs have been reported, such as programmed death-ligand 1 (PD-L1) high expression in tumors, high tumor mutation burden, and the accumulation of tumor-infiltrating lymphocytes in the tumor microenvironment (7,13). Of these, PD-L1 expression in tumors has been clinically used as a positive predictive biomarker for effective ICI treatment in NSCLC. High PD-L1 expression in tumors has been reported to suppress the activation and growth of T cells via the apoptosis of effector T cells, which in turn leads to impaired tumor immune responses (14,15). Thus, PD-L1 has been recognized as a negative regulator of the immune response through various pathways in anti-tumor immunity. Contrastingly, PD-L1 expression in tumors was reported as a negative predictive factor for response to Doc in NSCLC patients (5).
The aim of this retrospective study was to elucidate the role of PD-L1 expression in tumors and to identify responders for the treatment of Doc in combination with Ram in advanced or relapsed NSCLC patients who are refractory to platinum-based chemotherapy.
Methods
Patients
We retrospectively assessed advanced or relapsed 135 NSCLC patients who were refractory to platinum-based chemotherapy and had the evaluable targeted lesions, at 11 institutions in Japan between July 2016 and November 2018. All patients underwent image evaluation using a conventional computed tomography (CT) scan or a magnetic resonance imaging according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The PFS and OS were calculated from the beginning of Doc/Ram combination therapy. The following clinical data were obtained from retrospective medical records: age, sex, smoking status, histological subtype, PD-L1 tumor proportion score (TPS), disease stage, Eastern Cooperative Oncology Group Performance Status (PS), PFS, ORR, and the history of immunotherapy treatment before and after Doc plus Ram combination therapy. The study protocol was approved by the Ethics Committees of each hospital.
Genomic analysis
EGFR mutations were detected using polymerase chain reaction for tumor samples with the sequencing of exons 18–21 performed at commercial clinical laboratories; SRL, Inc. and BML, Inc. (Tokyo, Japan). ALK IHC staining using tissue specimens was performed using the Ventana platform (Roche Diagnostics, Basel, Switzerland) at commercial clinical laboratories; SRL, Inc. and BML, Inc. (Tokyo, Japan).
PD-L1 expression analysis
PD-L1 TPS expression in tumors was assessed in pretreatment tumor samples obtained from biopsy or surgery by performing PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay at commercial clinical laboratories in SRL, Inc. (Tokyo, Japan). The PD-L1 TPS was expressed as the percentage of at least 100 viable tumor cells for complete or partial membrane staining. The pathologists of the commercial vendor provided the TPS interpretation. The patients were categorized into the following three patient groups based on the PD-L1 expression: positive (TPS ≥1%), negative (TPS <1%), and unknown groups.
Treatment
Patients were intravenously administered with 10 mg/kg Ram plus 60 mg/m2 Doc on day 1 of a 3-week cycle. In general, these treatments continued until disease progression, intolerable toxicity, or patient refusal.
Matching
We performed rigorous adjustment for significant differences in the baseline characteristics of patients with propensity-score matching using the following variables: age, sex, PS, disease stage, cell type, smoking status, and treatment line of the initiation of the combination therapy of Doc and Ram. Nearest neighbor matching was performed at a ratio of 1:1 without replacement. Caliper was set at 0.2.
Statistical analysis
Cox proportional hazards models evaluating several patient factors were used. To analyze PFS, times to events were estimated using the Kaplan-Meier method and compared using the log-rank test. PFS was confirmed at the date of disease progression. To construct the multivariate model, we selected the factors related to PFS and OS that were reported by the previous studies and the most relevant factor identified in the results of univariate analysis. The statistical analyses except for the propensity score analysis were performed using Prism (version 8.01; GraphPad Software Inc, CA, USA). The propensity score analysis was performed using EZR for Windows, version 1.35 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 135 advanced NSCLC patients from Japan, who showed resistance to platinum-based chemotherapy were enrolled in the present study (Table 1). Ninety-five (70.4%) patients were male, the median age was 66 years (range, 37–83 years), and 92 (68.1%) patients had a history of smoking. Among them, 21 (15.6%) patients had squamous cell carcinoma and 118 (87.4%) patients had stage IV cancer. EGFR mutation and ALK rearrangement tests were performed on 119 (88.1%) and 108 (80.0%) patients, respectively. The tumor samples of 27 (20.0%) patients showed EGFR-mutation, while 2 (1.5%) showed ALK rearrangement. Almost all patients (92.6%) had PS of 0 or 1 at the time of the induction of the treatment with DOC and Ram. Patients received 3.0 (range, 1.0–14.0) cycles of the combination therapy of Doc and Ram.
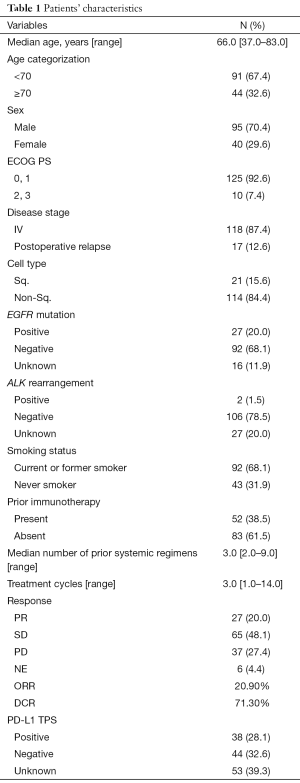
Full table
Relationship between tumor PD-L1 expression and clinicopathological features
The PD-L1 IHC test was performed in 82 (60.7%) out of the 135 NSCLC patients. Of them, 38, 44, and 53 patients were classified under the positive, negative, and unknown groups, respectively. The different patient groups showed no significant differences in clinicopathological features (Table 2).
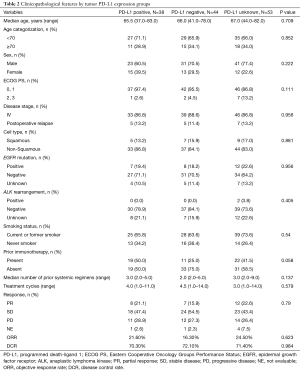
Full table
Relationship between treatment with Doc plus Ram response and PD-L1 expression
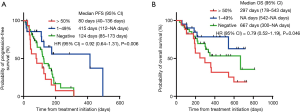
The ORR values of the treatment with Doc plus Ram were 21.1%, 15.9%, and 24.5% in NSCLC patients with PD-L1 positive, negative, and unknown status, respectively. The three patient groups showed no significant differences in the ORR values following treatment with Doc plus Ram (P=0.623). Median PFS with the treatment of Doc plus Ram was 127 days in all NSCLC patients (Figure 1A). Median OS was 359 days in all NSCLC patients (Figure 1B). There was no significant correlation in the median PFS among the NSCLC patients with PD-L1 positive, negative, and unknown status (127, 124, and 172 days, respectively, P=0.444) (Figure 1C). We observed no significant correlation in the median OS of the treatment among NSCLC patients with PD-L1 positive, negative, and unknown status (542, 667, and 299 days, respectively, P=0.154) (Figure 1D). To minimize the impact of treatment allocation bias, we performed the propensity score analysis for PD-L1 detectable and unknown cases. There was no significant correlation in the median PFS and OS between PD-L1 detectable and unknown cases (128 and 172 days, respectively, P=0.649; 349 and 299 days, respectively, P=0.328) (Figure 1E,F). We further examined the relationship between PD-L1 expression levels and clinical outcomes, such as the median PFS and OS of Doc plus Ram treatment (Table S1). The median PFS and OS were better in patients with 1–49% of PD-L1 expression than in those with negative and >50% of PD-L1 expression (P=0.006, P=0.046, respectively) (Figure S1A,B).
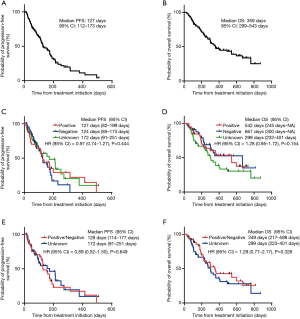
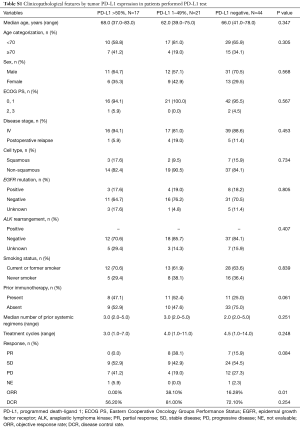
Full table
In the univariate analysis, the PFS for Doc plus Ram was relatively long in patients who received previous immunotherapy treatment compared to those of patients who did not receive prior immunotherapy, although there was not statistically significance between the two groups of patients [156 days, 95% confidence interval (CI): 105–314 vs. 116 days; 95% CI: 100–177 days; hazard ratio (HR): 0.63; 95% CI: 0.39–1.02, P=0.059] (Table 3). Results of multivariate analysis demonstrated that prior immunotherapy was an independent prognostic factor for prolonged PFS with Doc plus Ram treatment (HR: 0.59; 95% CI: 0.35–0.98, P=0.041) and poor ECOG PS was inversely an independent prognostic factor for prolonged OS (HR: 2.25; 95% CI: 1.01–5.01, P=0.047) (Table 4). A significant difference in the PFS of patients treated with Doc plus Ram was observed between patients who received prior immunotherapy and those who did not. This was determined based on the results of matched-pair analysis of PD-L1 expression levels and number of previous treatment lines (172 and 116 days, P=0.012) (Figure S2A). In contrast, there was no significant difference in OS between the two groups (401 days and not available, P=0.135) (Figure S2B).
Full table
Full table
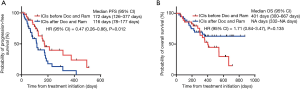
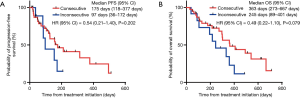
Next, we examined the timing of the combination treatment with Doc plus Ram after immunotherapy. Of the 52 patients who received immunotherapy prior to combination therapy with DOC and Ram, 40 patients treated by Doc plus Ram consecutively after immunotherapy. Remaining 12 patients had other treatment between immunotherapy and Doc plus Ram. Although the two groups showed no significant differences in median PFS and OS, patients treated with Doc and Ram consecutively after immunotherapy tended to have longer PFS and OS compared to those with inconsecutive administration of Doc plus Ram (PFS; HR: 0.54; 95% CI: 0.21–1.40, P=0.202, OS; HR: 0.49; 95% CI: 0.22–1.10, P=0.079, respectively) (Figure 2A,B).
Discussion
Current clinical trials demonstrated that combination regimens of chemotherapy and ICIs lead to better patient outcomes compared to chemotherapy alone as the first line of treatment with NSCLC, regardless of PD-L1 expression in tumors (16-18). Based on the results of phase III clinical trials, when patients show refractory responses to the initial systemic treatment, the combination of Doc plus Ram is recommended as the next line of treatment for NSCLC patients without driver oncogene alterations (10). However, the effectiveness of promising clinical biomarkers for predicting the efficacy of treatment with anti-angiogenesis agents, such as Ram, remains unclear in NSCLC patients. Thus, there is an urgent need to identify predictive biomarkers for positive response to combination therapy with Doc plus Ram.
To the best of our knowledge, the present study is the first to identify the clinical association between PD-L1 expression in tumors and the efficacy of combination therapy of Doc plus Ram in previously treated NSCLC patients. Besides immunotherapy, PD-L1 expression in tumors has been reported to be a negative predictor of clinical outcome in various malignancies (19-21), including positive response to Doc for previously treated NSCLC patients (5). However, our findings showed that there was no significant difference in OS between PD-L1-negative and PD-L1-positive or unknown status patients. In addition, PD-L1 expression in tumors was not associated with the efficacy of combined therapy of Doc plus Ram, which indicated that PD-L1 expression in tumors is not a negative mediator of clinical outcome to combination therapy in NSCLC patients. These findings suggested that PD-L1 expression in tumors are not critical for patients receiving combination therapy with Doc plus Ram. In addition, following treatment with the combination of Doc plus Ram, NSCLC patients with known PD-L1 expression status showed similar positive response rates to patients with unknown PD-L1 expression status. These encouraging results led us to hypothesize that combination therapy is a promising second-line standard treatment for NSCLC patients with unknown PD-L1 expression levels after refractory response to platinum-based chemotherapy. However, our observations showed that patients with 1–49% of PD-L1 expression had better clinical outcomes than those with negative and >50% of PD-L1 expression. Therefore, additional investigations are required to clearly understand the relationship between PD-L1 expression levels and clinical outcomes of Doc plus Ram treatment.
Regulatory T cells (Tregs) are known as potent biomarkers and prognostic factors for various malignancies (22,23). Recent studies showed that the frequency of Tregs in tumor microenvironments is a reliable biomarker for clinical responses to therapies with Ram, thereby suggesting that Ram can be an effective immunomodulator when administered in combination with immune checkpoint blockers (24). PD-L1 expression is associated with FOXP3-expressing Treg infiltration in tumors and poor prognosis in soft tissue sarcoma (25). Our findings suggested that treatment with Doc in combination with Ram can be effective for the treatment of PD-L1-expressing NSCLC tumors by modulating the tumor microenvironment. Therefore, this combination therapy could be more effective for PD-L1-expressing NSCLC patients than treatment with Doc alone. However, further studies are needed to validate our observations.
To further investigate the therapeutic biomarkers for Doc plus Ram, we analyzed responders of treatment with Doc plus Ram from the point of view of clinical profiles in previously treated NSCLC patients. Among several clinical parameters, prior treatment with ICIs was found to be a good predictor for a positive response to Doc plus Ram in NSCLC patients, and this was confirmed using the matched-pair analysis.
Consistent with our findings, previous studies showed that prior nivolumab treatment was associated with a high response rates to consecutive administration with Doc plus Ram after nivolumab (26). Results indicated that nivolumab continued to bind PD-L1 for approximately two months and then stopped, indicating that inhibition of PD-L1 activity by prior treatment with ICIs was maintained when subsequent treatment was administered (27,28). Our results demonstrated that treatment with Doc plus Ram with consecutive administration of ICIs led to better patient outcomes compared to inconsecutive administration with ICIs. These observations supported that immunotherapy followed by Doc plus Ram is a promising sequential strategy for PD-L1 regulation. Further studies are warranted to evaluate our observations regarding the efficacy of sequential strategy with Doc plus Ram.
The present study has several limitations. First, it is a retrospective study, and the cohort had a limited sample size of 130 cases. Second, all patients in the cohort were Japanese. Third, the study has several biases on patient conditions when treatment with Doc in combination with Ram started, such as the number of pre-treatment regimens and the PS of patients. Fourth, the frequency of PD-L1 detection is limited. However, although the study was retrospective, our novel findings regarding patient response to this combination therapy are notable and could be useful for addressing clinical issues. Future prospective investigations are warranted to verify our findings.
Conclusions
We retrospectively showed that PD-L1 expression in tumors is not significantly associated with the efficacy of combined therapy with Doc plus Ram in previously treated 135 NSCLC patients. However, results indicated that prior treatment with ICIs is a reliable predictor for good response to Doc plus Ram. Our observations suggested that combination regimens with chemotherapy and ICIs followed by Doc plus Ram could serve as optimal therapeutic options for NSCLC patients without driver oncogene alterations, regardless of PD-L1 status in tumors. Further studies are required to accumulate clinical evidence demonstrating the effectiveness of combination therapy with Doc plus Ram for previously treated NSCLC patients.
Acknowledgments
We thank the patients, their families, and all investigators involved in this study. We are also grateful to Rumi Makino and Hiroko Tamaru for assisting with the administrative work and Dr. Masahiro Iwasaku for supporting statistical analysis.
Footnote
Conflicts of Interest: Dr. Yamada reports receiving research grants from Pfizer Inc., Boehringer Ingelheim Japan Inc., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co. Ltd., and Chugai Pharmaceutical Co., Ltd. Dr. Uchino reports receiving research grants from Eli Lilly Japan K.K., AstraZeneca K.K., and Boehringer Ingelheim Japan Inc. Dr. Takayama reports receiving research grants from Chugai-Roche Co., and Ono Pharmaceutical Co., and personal fees from AstraZeneca Co., Chugai-Roche Co., MSD-Merck Co., Eli Lilly Co., Boehringer-Ingelheim Co., and Daiichi-Sankyo Co. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committees of Kyoto Prefectural University of Medicine (No. ERB-C-1336-1) and each hospital, and conducted in accordance with the principles of the Declaration of Helsinki.
References
- Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin 2018;68:425-45. [Crossref] [PubMed]
- Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer 2015;87:193-200. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Passiglia F, Galvano A, Rizzo S, et al. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: An indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer 2018;142:1277-84. [Crossref] [PubMed]
- Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer 2018;115:12-20. [Crossref] [PubMed]
- Hatam LJ, Devoti JA, Rosenthal DW, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res 2012;18:1925-35. [Crossref] [PubMed]
- Wenjin Z, Chuanhui P, Yunle W, et al. Longitudinal fluctuations in PD1 and PD-L1 expression in association with changes in anti-viral immune response in chronic hepatitis B. BMC Gastroenterol 2012;12:109. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Gadiot J, Hooijkaas AI, Kaiser AD, et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011;117:2192-201. [Crossref] [PubMed]
- Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol 2014;25:2178-84. [Crossref] [PubMed]
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361-70. [Crossref] [PubMed]
- Jang TJ. Progressive Increase of Regulatory T Cells and Decrease of CD8+ T Cells and CD8+ T Cells/Regulatory T Cells Ratio during Colorectal Cancer Development. Korean J Pathol 2013;47:443-51. [Crossref] [PubMed]
- Pan XD, Mao YQ, Zhu LJ, et al. Changes of regulatory T cells and FoxP3 gene expression in the aging process and its relationship with lung tumors in humans and mice. Chin Med J (Engl) 2012;125:2004-11. [PubMed]
- Tada Y, Togashi Y, Kotani D, et al. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8(+) T cells in the tumor microenvironment. J Immunother Cancer 2018;6:106. [Crossref] [PubMed]
- Que Y, Xiao W, Guan YX, et al. PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis. J Cancer 2017;8:2018-25. [Crossref] [PubMed]
- Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 2019;10:775-81. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res 2015;21:4286-93. [Crossref] [PubMed]


