
Management of local recurrences and regional failure in early stage non-small cell lung cancer after stereotactic body radiation therapy
Introduction
Stereotactic body radiation therapy (SBRT) is the standard of care in early-stage non-small cell lung cancer (NSCLC) for medically inoperable patients. SBRT has a 5-year infield control of over 90% and a 5-year loco-regional control of approximately 75% (1). In defining cancer specific outcomes, patterns of recurrence become important. We define local recurrence as being at the site of treatment (within the initial PTV), regional failure as being in the chest outside the treated PTV in the same lobe of the lung or mediastinal nodal failures.
Post-SBRT true local recurrences are rare, but unfortunately do occur (optimally ≤5%). Factors contributing to local failure are multiple. Often not acknowledged is that, unlike a lobectomy which results in loss of significant volume of lung tissue, SBRT maintains the patient lung volumes and patients actually may have more lung volume than surgically treated patients in which to fail. Similarly, it appears that those patients who continue to smoke have a high risk of developing new lung cancers (2). Radiation dose, gross tumor volume, fraction size, total dose and prescription (for example isocenter versus volumetric) may affect incidence of local failures (3). Thus, like any cancer therapy, salvage for a recurrence after SBRT may become necessary. It is important to note and often overlooked that the technique and results of SBRT treatment do not preclude salvage if needed. In this review we will attempt to address the various strategies which may be taken to address the rare local failures post-SBRT.
There is evidence that even commonly used doses such as 48–50 Gy in 4–5 fractions and 50–60 Gy in 8–10 fractions may have inferior local control compared to higher biologically effective dose regimens like 54 Gy in 3 fractions (4). This may be especially true for squamous cell carcinoma (5). However, true failure rates for patients with adenocarcinoma may be unknown if patients have not been followed long term because adenocarcinoma may have a longer time to failure. A retrospective study by Shintani et al. investigated the incidence and time course of late local control. This study looked at tumors which were under 3 cm and were treated with 48 Gy in 4 fractions prescribed to the isocenter. The 5-year actuarial local control rate was 81%. They found the median time to local recurrence was 1.3 years for squamous cell carcinoma and 2.1 years for adenocarcinoma, but recurrences were still possible after 5 years (6).
In order to search for appropriate studies, PubMed, the Cochrane Library, and Google Scholar were all queried for studies that included SBRT as the primary treatment for lung lesions which then recurred and required salvage therapy. Eligible studies included those that were used for either early-stage NSCLC or metastatic lesions. The primary therapy had to fit the conventional definition of stereotactic treatment which is a method of external beam radiotherapy (EBRT) that uses highly precise localization of the target to deliver a high dose of radiation (usually 600 cGy or more per fraction) in a few fractions to an extracranial target.
Results
Both surgery and reirradiation are strategies that have been used after a post-SBRT recurrence. A group of six radiation oncologists in different institutions across the world were given a hypothetical scenario in which a patient had received SBRT and experienced a relapse at the primary site and/or mediastinum. Half of the experts recommended surgery primarily for salvage and all of the experts stated that the prior SBRT did not exclude the patient from receiving definitive chemoradiation for locally advanced recurrent disease (7). In retrospective studies, both surgery and re-irradiation have shown promising results. A few studies have combined the outcomes of both techniques.
In a study by Hamamoto et al., the authors retrospectively reviewed 90 patients who were treated with SBRT for early stage NSCLC. Twenty-seven of the patients had lung-cancer-related chest events including local failure, regional failure, additional lung metastases, pleural disseminations and/or new primary lung cancers. These events were detected at a median follow up of 26 months. Regarding the 10 local failures, 70% of them were able to be treated with curative-intent therapy. Five were treated with salvage SBRT and two with salvage surgery. Those that received curative intent salvage therapy of either modality had a significantly better 1-year overall survival (OS) of 86% compared to a 1-year OS of 40% for those that did not (P=0.0289) (8). In another investigation, Brooks et al. retrospectively examined the charts of 912 T1N0 NSCLC patients salvaged by surgery or reirradiation. One hundred and two of these patients developed isolated local and/or regional failures, with 49 being isolated local failures (5.4%) and 46 being isolated regional failures (5.0%). Approximately 80% of the patients with isolated local failures and 90% of the patients with isolated regional failures went on to receive further therapy with several different modalities. In patients with isolated local recurrence, the patients were treated with repeated SBRT or surgery. Fifteen patients were treated with salvage SBRT and 10 patients were treated with salvage surgery. One out of 15 patients treated with salvage SBRT and four out of 10 patients treated with surgery experienced Grade 3 or higher side effects Conversely, over half of the patients with isolated regional failures (n=26) were treated with chemoradiotherapy, with ten of the 26 experiencing Grade 3 or higher toxicity. There was no Grade 5 toxicity. They found that in these patients, survival was significantly longer for patients who received salvage therapy, 37 vs. 7 months. They also found that patients with a salvaged isolated regional failure had a statistically similar 5-year OS to those that had never recurred (57.9% vs. 54.9%), but those with a salvaged isolated regional recurrence only had a 5-year OS of 31.1%, which is comparable to patients with stage III NSCLC. Approximately 50% of those with a salvaged isolated local recurrence and 70% with a salvaged isolated regional recurrence had no further recurrences. Most recurrences were regional and distal recurrences for both groups (9).
Surgery
Salvage surgery, if possible, is a safe and effective way to salvage post-SBRT local recurrences. Ten such studies were found, and findings are summarized in Table 1. Some concerns with surgery are fibrosis, adhesions, and more difficult operations due to the changes in the lung and surrounding tissues after SBRT. The main issue with SBRT combined with surgery may be the result of the proximity of the target volumes near critical structures such as the pleura or hilum which needs to be taken into account by the appropriate pre-op surgical assessment. More prospective data on this issue is needed. The feasibility of salvage surgery after definitive SBRT for early-stage lung cancer was explored in several retrospective studies. Two of these were published in 2010. In these studies, 5–7 patients who had received prior SBRT then went on to have either a lobectomy or segmentectomy in the same lobe. None of the patients had pleural adhesions in the lungs and surgery was seen as a feasible option (10,11). Other studies have shown a few limited toxicities. Another study by Allibhai was presented in 2012. Four patients with isolated local recurrences after SBRT were identified and underwent salvage surgery. There were no significant intraoperative difficulties or post-operative complications. All four patients were alive at a median follow up of 30 months (12). Complications during or after surgery certainly can and do happen, though. Hamaji et al. looked at twelve similar post-surgical salvage patients who developed two intrapleural adhesions and three patients who experienced air leaks after 5 days post-operatively (13). There is also a follow-up case report by the same group in which SBRT failures for oligometastatic disease were salvaged with lobectomies. There were 3 patients retrospectively examined in this study. No patients had any intraoperative or postoperative complications. At 6, 40, and 51 months of follow-up no patient had recurred. Once again, the conclusion was that surgical salvages were feasible and safe (14). An additional retrospective study examined 21 patients who underwent salvage surgery after a local failure post-SBRT. Seven patients experienced postoperative complications, two with atrial arrhythmias and two with prolonged air leaks. There were no local recurrences after surgery. Median OS was 46.9 months and 3-year survival was 71.8% (15). A study by Verstegen et al. examined 9 patients who had a local failure after SBRT. Five had salvage surgery and four had salvage RT. In those that had surgery 77% of the patients had limited or no adhesions. There was only one patient with a persistent air leak and the paper concluded that salvage surgery was safe after SBRT. Unfortunately, one of nine patients died within 90 days, but this was due to progressive disease. The median OS after salvage therapy was 36 months (16).
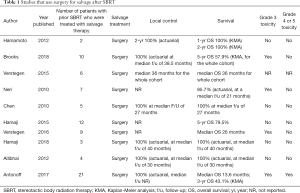
Full table
In a separate small report, two patients underwent salvage operations for isolated local recurrences detected on computer tomography imaging. Both of the patients had no viable tumor cells in the resected surgical specimens, highlighting another important issue: the difficulty of distinguishing local recurrence from post-treatment changes on imaging studies (17).
Comparisons have been made between those patients who receive surgery and those that do not for a post-SBRT local recurrence. Hamaji et al. performed a retrospective analysis on SBRT patients comparing salvage surgery with best supportive care. Of those with isolated local failures, the ones that were treated with salvage surgery had an improved median OS compared to those treated with best supportive care alone of 25.0 vs. 10.5 months. Additionally, those treated with salvage surgery had a significantly improved 5-year OS as well (47.9% vs. 31.5%). Interestingly, this paper also looked at a population of patients who received chemotherapy alone as salvage therapy; the 5-year OS was 0% (13).
Radiation
Whenever considering reirradiation to the same thoracic site, it is of the utmost importance to limit dose to the organs at risk such as the spinal cord, heart, esophagus, surrounding lung, and other tissues. Despite the risks that reirradiation can pose, there are several studies which show reirradiation for local failures can sometimes be done with minimal toxicities. After prior SBRT, reirradiation can be delivered as a second course of SBRT or conventionally fractionated external beam radiotherapy (CF-EBRT). With careful planning and patient selection, both techniques have proven to be safe and effective.
Conventionally fractionated EBRT
Though there are more published studies regarding the use of SBRT in the recurrent setting, there are some studies that examine the use of salvage CF-EBRT after a post-SBRT local failure. Four such studies were found, and findings are summarized in Table 2. Usually CF-EBRT is utilized in patients with regional failures such as mediastinal or hilar disease. In the absence of a local failure, it is possibly less of a failure of the SBRT itself, and more of a failure to properly select for the patient’s without nodal disease. Unfortunately, regional failures can occur in 5–15% of patients with early stage NSCLC who are treated with SBRT. Salvage can be done with CF-EBRT with or without the use of chemotherapy.
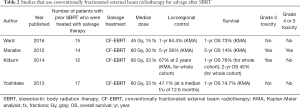
Full table
The issues regarding isolated nodal failures were addressed in a Cleveland Clinic study. They found that 3% of their patients had an isolated nodal failure and 63% of these patients were able to receive salvage nodal radiation. The most common regimen was 45 Gy in 15 fractions. Those who received radiotherapy had longer 1-year OS (73% vs. 56%) and 1 year progression free survival (75% vs. 33%) than those that did not. After salvage mediastinal radiotherapy the 1-year locoregional control was 84.4%. No patients experienced Grade 3 or higher toxicity (18). In a retrospective study, Manabe et al. identified patients with hilar or mediastinal recurrences after SBRT or surgery. For those patients treated with SBRT, they received a median of 60 Gy to the mediastinum or hilum. Only one patient in this group received chemotherapy. Those treated with SBRT and then mediastinal EBRT had a 5-year OS of 14%, a 5-year progression free survival of 21%, and a 5-year local control of 58%. This study notes that four of the fourteen original SBRT patients survived over three years after salvage therapy (19). A similar study was conducted by a group at Wake Forest. Twelve patients were identified with isolated nodal recurrences, only two of which received chemotherapy. Median salvage dose was 66 Gy. Median OS after salvage was 15 months (20).
Chemotherapy is also often used in the salvage setting with CF-EBRT. A paper by Yoshitake et al. retrospectively evaluated seventeen patients treated with chemoradiotherapy following a local failure after SBRT. The radiation was standard fractionation EBRT delivered as 60 Gy in 30 fractions. Four of these patients had regional and/or distant metastases at the time of retreatment. 52.9% of patients experienced a local recurrence. At one year local PFS was 33.8% and OS was 74.7%. There were no Grade 3 or higher adverse events (21). A paper by Kilburn et al. about reirradiation of lung tumors included a population of patients that had been treated initially with SBRT. Seven patients were salvaged with a repeat SBRT and three were salvaged with CF-EBRT. Those that were salvaged with CF-EBRT as opposed to SBRT were done so due to mediastinal or hilar recurrences. Seventy percent of these patients were treated with chemotherapy. This study also included those who were initially treated with EBRT and then had a local recurrence that was treated with SBRT. Two-year local control was 67% and those that were treated with a single fraction of 20–22.5 Gy were more likely to fail than others. The study found that the median OS for the entire group was 21 months with a 1-year OS of 76% and a 2-year OS of 45%. In those that were initially treated with SBRT and then salvaged with EBRT or SBRT, there were no Grade 4 or 5 toxicities (22).
SBRT
There is more data on the use of SBRT for local failures than the use of CF-EBRT. Local failures could occur for many reasons after prior SBRT, including marginal misses. Thus, it stands to reason that if the organs at risk can be properly protected, another course with an ablative dose and fractionation could be beneficial. Repeat SBRT has been used with caution as there can be poor outcomes, but if proper care is taken to limit dose to the normal structures, it has been shown to be safe and feasible. Seventeen such studies were found, and findings are summarized in Table 3. There have been a few SBRT retreatment studies which mix a small number of patients with prior SBRT with a larger number of prior EBRT patients and do not distinguish between the outcomes of the two groups (23-26). All of these studies have small numbers, but conclude that it is possible for re-irradiation with SBRT to be done safely and effectively.
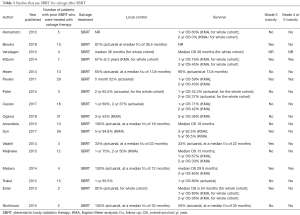
Full table
It is very possible to harm patients with inappropriate patient/tumor selection and poor dose selection. A Swedish retrospective review identified 29 patients with prior SBRT who had suffered from a local failure. These patients were retreated with another course of SBRT. Most of these patients had metastatic disease and 72% of the population were treated with SBRT for other lung tumors. One third had centrally-located tumors within 2 cm of the tracheobronchial tree. This study resulted in higher than acceptable toxicity. Two patients had Grade 4 toxicity and 3 patients had Grade 5 toxicity. All patients with Grade 4 or 5 toxicity had centrally located tumors. There were three patients who suffered from Grade 5 toxicity (hemoptysis). In one case, the patient did not receive a curative dose initially and an autopsy was not performed. In another, SBRT was given twice to the bilateral hila and resulted in bronchial stenosis and hemoptysis. In the third case, the patient received two courses of SBRT to the hilum and suffered from fatal hemoptysis. The toxicity may be due partially to the fact that a 4D CT was not used and thus there were large margins (up to 2 mm CTV and 10 mm PTV). Notably, not all tumors were treated with a BED10 of >100 Gy with each SBRT treatment. At 5 months, local control was achieved in 52% of patients. Using a Kaplan Meier estimate, median OS was 19.3 months and 1-, 2-, and 3-year OS were 59%, 43%, and 23%, respectively (27). If the dose constraints can be met appropriately, appropriate dose and BED prescriptions are also critically important. A Temple University retrospective study identified three patients that had an in-field recurrence after initial SBRT. These patients were again re-treated with SBRT to a median dose of 60 Gy. Two of them again experienced recurrences and eventually died due to their disease, one of the two received a BED 10 <100 Gy with both courses. There were no Grade 4 or 5 toxicities (28).
Despite these results, repeat irradiation with SBRT after prior SBRT can be done safely. A retrospective study from Stanford by Trakul et al. identified 4 patients that had tumors that were local recurrences after prior SBRT and treated with subsequent salvage SBRT. Three of the 4 patients remained controlled locally and distantly after a median follow up of 15 months (29). Ester et al. reported two patients that were treated with prior SBRT that experienced a local recurrence. Both were treated with repeat SBRT. At a median of 14 months one of the two was alive and without evidence of disease. There were no Grade 4 or 5 toxicities (30). A small study by Nishimura et al. showed similar results (31). A small retrospective study done at the University of Louisville examined eighteen patients with local or regional recurrence after SBRT. Ten were treated with repeat SBRT, six with platinum-based chemotherapy alone, and two with palliative measure alone. The patients treated with chemotherapy or palliative measures tended to also have distant disease. Four of the patients treated with repeat SBRT were treated for infield recurrences. There were no Grade 4 or 5 toxicities (32). A Cleveland Clinic retrospectively reviewed 22 patients with isolated local failure. Ten of these patients received SBRT as salvage therapy. This study noted that several patients were deemed ineligible for repeat SBRT due to tumor size, proximity to the tracheobronchial tree, extensive abutment, among other reasons. No patient had tumors within 2 cm of the tracheobronchial tree. All patients were treated to a BED10 ≥100 Gy. There was no Grade 3 or higher toxicity experienced by these patients. They noted that at the time of publication, with a follow-up of at least 11.7 months, three patients were still alive and without evidence of disease (33). Similarly, a study by Meijneke et al. examined 14 patients that were previously treated with SBRT with local recurrences. Twelve of them received curative-intent SBRT, one received curative-intent conventional EBRT, and one received palliative-intent conventional EBRT. Using an α/βb ratio of 3, the median V20 was 15.2% and the median MLD was 15 Gy. There was no Grade 3 or higher toxicity noted. Median OS was 15 months and 1- and 2-year OS was 67% and 33%, respectively. One-year LC was 75% and 2-year LC was 50% (34). Reirradiation to central tumors can be accomplished with careful selection of patients and doses. A recent Japanese study published in 2018, identified thirty-one patients with prior lung SBRT and were treated with re-irradiation. Half of the recurrences were biopsy-proven. Nine patients had centrally located tumors, but none abutting the central airways. The initial SBRT dose was treated with 48–52 Gy in 4 fractions and the second course was treated similarly or with 60 Gy in 8 fractions. Three-year OS and local control were 36% and 53%, respectively. Four patients survived greater than five years with no further evidence of local disease. There was no Grade 3 or higher toxicity observed (35).
Conclusions
Unfortunately, like every cancer therapy both local recurrences and regional or metastatic failure can occur after SBRT for lung tumors. This mandates the use of high-quality PET/CT for staging and use of high biological effective dose SBRT usually prescribed to cover the planning target volume (PTV) rather than isocenter. Nonetheless, both surgery and repeat SBRT can be safe and effective for local recurrences in the lung. Both must be used with caution and eligible patients must be selected with care. Strategies employing standard of care CF-EBRT is generally used in cases where there is mediastinal/hilar nodal disease or metastasis. This salvage therapy can be used both with and without chemotherapy. As the use of immunotherapy moves forward, strategies to incorporate it, perhaps upfront or post-SBRT, need to be examined.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [Crossref] [PubMed]
- Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res 2017;6:131-47. [Crossref] [PubMed]
- Stephans KL, Woody NM, Reddy CA, et al. Tumor Control and Toxicity for Common Stereotactic Body Radiation Therapy Dose-Fractionation Regimens in Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:462-9. [Crossref] [PubMed]
- Shiue K, Cerra-Franco A, Shapiro R, et al. Histology, Tumor Volume, and Radiation Dose Predict Outcomes in NSCLC Patients After Stereotactic Ablative Radiotherapy. J Thorac Oncol 2018;13:1549-59. [Crossref] [PubMed]
- Shintani T, Matsuo Y, Iizuka Y, et al. A Retrospective Long-term Follow-up Study of Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer From a Single Institution: Incidence of Late Local Recurrence. Int J Radiat Oncol Biol Phys 2018;100:1228-36. [Crossref] [PubMed]
- Nieder C, De Ruysscher D, Gaspar LE, et al. Reirradiation of recurrent node-positive non-small cell lung cancer after previous stereotactic radiotherapy for stage I disease. Strahlenther Onkol 2017;193:515-24. [Crossref] [PubMed]
- Hamamoto Y, Kataoka M, Yamashita M, et al. Lung-cancer related chest events detected by periodical follow-up CT after stereotactic body radiotherapy for stage I primary lung cancer: retrospective analysis of incidence of lung-cancer related chest events and outcomes of salvage treatment. Jpn J Radiol 2012;30:671-5. [Crossref] [PubMed]
- Brooks ED, Sun B, Feng L, et al. Association of Long-term Outcomes and Survival With Multidisciplinary Salvage Treatment for Local and Regional Recurrence After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. JAMA Netw Open 2018;1:e181390. [Crossref] [PubMed]
- Neri S, Takahashi Y, Terashi T, et al. Surgical Treatment of Local Recurrence After Stereotactic Body Radiotherapy for Primary and Metastatic Lung Cancers. J Thorac Oncol 2010;5:2003-7. [Crossref] [PubMed]
- Chen F, Matsuo Y, Yoshizawa A, et al. Salvage Lung Resection for Non-small Cell Lung Cancer After Stereotactic Body Radiotherapy in Initially Operable Patients. J Thorac Oncol 2010;5:1999-2002. [Crossref] [PubMed]
- Allibhai Z, Cho BCJ, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J 2012;39:1039-42. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Hamaji M, Mitsuyoshi T, Yoshizawa A, et al. Salvage Pulmonary Metastasectomy for Local Relapse After Stereotactic Body Radiotherapy. Ann Thorac Surg 2018;105:e165-8. [Crossref] [PubMed]
- Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154:689-99. [Crossref] [PubMed]
- Verstegen NE, Lagerwaard FJ, Hashemi SMS, et al. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J Thorac Oncol 2015;10:1195-200. [Crossref] [PubMed]
- Taira N, Kawabata T, Ichi T, et al. Salvage Operation for Late Recurrence After Stereotactic Body Radiotherapy for Lung Cancer: Two Patients With No Viable Cancer Cells. Ann Thorac Surg 2014;97:2167-71. [Crossref] [PubMed]
- Ward MC, Oh SC, Pham YD, et al. Isolated Nodal Failure after Stereotactic Body Radiotherapy for Lung Cancer: The Role for Salvage Mediastinal Radiotherapy. J Thorac Oncol 2016;11:1558-64. [Crossref] [PubMed]
- Manabe Y, Shibamoto Y, Baba F, et al. Definitive radiotherapy for hilar and/or mediastinal lymph node metastases after stereotactic body radiotherapy or surgery for stage I non-small cell lung cancer: 5-year results. Jpn J Radiol 2018;36:719-25. [Crossref] [PubMed]
- Kilburn JM, Lester SC, Lucas JT, et al. Management of mediastinal relapse after treatment with stereotactic body radiotherapy or accelerated hypofractionated radiotherapy for stage I/II non-small-cell lung cancer. J Thorac Oncol 2014;9:572-6. [Crossref] [PubMed]
- Yoshitake T, Shioyama Y, Nakamura K, et al. Definitive Fractionated Re-irradiation for Local Recurrence Following Stereotactic Body Radiotherapy for Primary Lung Cancer. Anticancer Res 2013;33:5649-53. [PubMed]
- Kilburn JM, Kuremsky JG, Blackstock AW, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT; techniques as first or second course of treatment. Radiother Oncol 2014;110:505-10. [Crossref] [PubMed]
- Patel NR, Lanciano R, Sura K, et al. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J Radiat Oncol 2015;4:65-70. [Crossref] [PubMed]
- Ceylan C, Hamacı A, Ayata H, et al. Re-Irradiation of Locoregional NSCLC Recurrence Using Robotic Stereotactic Body Radiotherapy. Oncol Res Treat 2017;40:207-14. [Crossref] [PubMed]
- Amendola BE, Amendola MA, Perez N, et al. Local failure after primary radiotherapy in lung cancer: Is there a role for SBRT? Rep Pract Oncol Radiother 2015;20:440-5. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki R, et al. Long-Term Outcomes of Salvage Stereotactic Ablative Radiotherapy for Isolated Lung Recurrence of Non-Small Cell Lung Cancer: A Phase II Clinical Trial. J Thorac Oncol 2017;12:983-92. [Crossref] [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [Crossref] [PubMed]
- Valakh V, Miyamoto C, Micaily B, et al. Repeat stereotactic body radiation therapy for patients with pulmonary malignancies who had previously received SBRT to the same or an adjacent tumor site. J Cancer Res Ther 2013;9:680-5. [Crossref] [PubMed]
- Trakul N, Harris JP, Le QT, et al. Stereotactic Ablative Radiotherapy for Reirradiation of Locally Recurrent Lung Tumors. J Thorac Oncol 2012;7:1462-5. [Crossref] [PubMed]
- Ester EC, Jones DA, Vernon MR, et al. Lung reirradiation with stereotactic body radiotherapy (SBRT). J Radiosurg SBRT 2013;2:325-31. [PubMed]
- Nishimura S, Takeda A, Sanuki N, et al. Dose-Escalated Stereotactic Body Radiotherapy (SBRT) as a Salvage Treatment for Two Cases with Relapsed Peripheral Lung Cancer After Initial SBRT. J Thorac Oncol 2015;10:e69-71. [Crossref] [PubMed]
- Mezera M, Chandrasekhar M, Kloecker G, et al. Evaluating patterns of failure and salvage therapy for patients treated with primary stereotactic body radiation therapy for early stage non-small cell lung cancer. Journal of Solid Tumors 2014. [Crossref]
- Hearn JWD, Videtic GMM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Meijneke TR, Petit SF, Wentzler D, et al. Reirradiation and stereotactic radiotherapy for tumors in the lung: Dose summation and toxicity. Radiother Oncol 2013;107:423-7. [Crossref] [PubMed]
- Ogawa Y, Shibamoto Y, Hashizume C, et al. Repeat stereotactic body radiotherapy (SBRT) for local recurrence of non-small cell lung cancer and lung metastasis after first SBRT. Radiat Oncol 2018;13:136. [Crossref] [PubMed]


