Clinical trials for lung cancer in China
Introduction
Chinese investigators have become increasingly active in lung cancer research in recent years. The Chinese Thoracic Oncology Group (CTONG) was established in 2007 with the purpose of designing and developing multi-center clinical trials to provide high-level evidence for lung cancer treatment in China. CTONG has launched 30 clinical trials that have contributed to or will contribute to establishing treatments for lung cancer patients. The ongoing trials are summarized in Table 1.
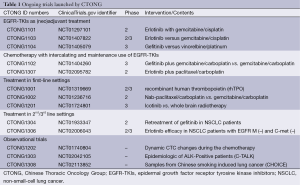
Full table
Completed trials led by Chinese investigators
Trials in a first-line settings
The IRESSA Pan-Asia Study (IPASS) enrolled patients in East Asia with advanced pulmonary adenocarcinoma who were nonsmokers or former light smokers to receive gefitinib or carboplatin plus paclitaxel (1). The trial showed that gefitinib was superior to carboplatin–paclitaxel in selected patients [hazard ratio (HR) for progression or death, 0.74; P
OPTIMAL (CTONG 0802) compared the efficacy and tolerability of erlotinib versus standard chemotherapy in patients with advanced EGFR-mutation-positive non-small-cell lung cancer (NSCLC) (2). The results showed that erlotinib conferred a significant progression-free survival (PFS) benefit (13.1 vs. 4.6 months; HR 0.16; P
FASTACT-2 (CTONG 0902) enrolled NSCLC patients with stage IIIB/IV disease and randomized them to receive gemcitabine plus platinum (GP) with intercalated erlotinib or placebo (3). The primary endpoint of PFS in the overall population was significantly longer for the chemotherapy plus erlotinib group than the chemotherapy plus placebo group (median PFS 7.6 vs. 6.0 months; Pvs. 6.9 months; Pvs. 20.6 months; P=0.0092). The results suggested that intercalated chemotherapy and erlotinib is a viable first-line option for NSCLC patients with EGFR-mutation-positive disease or selected patients with unknown EGFR mutation status.
LUX-Lung 6 is an international, phase III trial. Patients with an EGFR activating mutation were randomized to receive gemcitabine and carboplatin (GC) or afatinib (4). The primary endpoint, PFS, was significantly longer in the afatinib group than in the GC group (median 11.0 vs. 5.6 months; P
The BEYOND (NCT01364012) (5) study recruited advanced non-squamous NSCLC patients in China. They were randomized to receive carboplatin/paclitaxel plus bevacizumab or placebo. The primary endpoint, PFS, was prolonged in the carboplatin/paclitaxel plus bevacizumab arm (median 9.2 vs. 6.5 months; P
Chinese patients with stage IIIB/IV non-squamous NSCLC were enrolled in JMIL and assigned randomly to receive pemetrexed plus cisplatin (PC) or GC (6). The primary endpoint was to compare the OS in the combined dataset, consisting of Chinese patients in JMIL and 1,252 non-squamous patients in JMDB. The results showed that PC was superior to GC in terms of prolonging the OS in the combined dataset (median OS 11.76 vs. 10.94 months; P=0.023). Consequently, the investigators suggested that PC be considered as first-line treatment in Chinese non-squamous NSCLC patients.
Trials of maintenance treatment
INFORM (CTONG 0804, NCT00770588) enrolled patients from East Asia with advanced NSCLC who had not experienced disease progression or unacceptable toxicity during first-line standard platinum-based chemotherapy to receive gefitinib as maintenance (7). The primary endpoint PFS was significantly longer with gefitinib than with placebo (median PFS 4.8 vs. 2.6 months; HR 0.42; P
Trials in a second-line settings
The JMID (NCT00391274) study enrolled NSCLC patients who had prior chemotherapy and were randomized to receive single-agent pemetrexed or docetaxel until progressive disease or unacceptable toxicity developed or a patient/investigator decision was made (8). The primary endpoint of OS non-inferiority of pemetrexed to docetaxel was not met. The median OS was 11.7 and 12.2 months for the pemetrexed and docetaxel arms, respectively (P=0.492). Patients treated with pemetrexed had significantly fewer drug-related grade 3-4 adverse events (pemetrexed 20.8%, docetaxel 40.2%; P=0.003). Given the comparable efficacy and superior tolerability of pemetrexed, the investigators supported the use of single-agent, second-line pemetrexed for advanced non-squamous NSCLC in Chinese patients.
CTONG 0803 recruited patients with adenocarcinoma or an activating EGFR mutation and asymptomatic brain metastases (BM) without extracranial progressive disease after first-line platinum-doublet chemotherapy (9). The enrolled patients were given erlotinib. The median PFS was 10.1 months for intracranial progression and 9.7 months for intracranial and systemic progression. The trial suggested that single-agent erlotinib was active and well tolerated in NSCLC patients with BM.
CTONG 0806 recruited patients with advanced NSCLC without the EGFR mutation who had previously received platinum-based chemotherapy (10). The patients were randomized to receive pemetrexed or gefitinib. The primary endpoint PFS was significantly longer in the pemetrexed group than in the gefitinib group (median PFS 4.8 vs. 1.6 months; P
The ten trials mentioned above were representative, meaningful trials led by Chinese investigators. We want to emphasize the following three points: both gefitinib and erlotinib can be used for EGFR-mutant advanced NSCLC in China and erlotinib might be a better option for patients with BM; EGFR-tyrosine kinase inhibitor (EGFR-TKI) maintenance treatment is also a model for EGFR-mutant patients; and in a second-line or more settings, biomarker detection remains critical. Chemotherapy should be recommended for wild-type EGFR patients.
Ongoing trials led Chinese investigators
Trials of EGFR-TKIs as (neo)adjuvant treatment
Several trials have investigated the use of EGFR-TKIs as adjuvant treatment in early stage NSCLC. BR 19 was conducted in completely resected stage IB-IIIA NSCLC from 2003 to 2005 regardless of EGFR genotype (11). Unfortunately, adjuvant gefitinib did not confer a disease-free survival (DFS) or OS advantage in the overall population. The results of the SELECT and RADIANT trials raised widespread concern at the 2014 American Society of Clinical Oncology (ASCO) meeting (12,13). Both trials are of adjuvant erlotinib. The subgroup analysis of the RADIANT trial suggested that adjuvant erlotinib prolongs the DFS in patients with completely resected EGFR-mutated NSCLC, although not significantly so. SELECT was a phase II trial that suggested that patients treated with adjuvant erlotinib had an improved 2-year DFS.
Nevertheless, we are still unsure about the efficacy of (neo)adjuvant EGFR-TKIs based on the above-mentioned results. CTONG 1101, CTONG 1103, and CTONG 1104 should provide more evidence regarding this treatment strategy.
CTONG 1101 (NCT01297101) (14) is a phase II study of intercalated erlotinib with GC as neoadjuvant treatment in stage IIIA NSCLC patients. The disease control rate (DCR) was 92.3%. Five of the thirty-nine patients developed grade 3 common terminology criteria for adverse events (CTCAE). No one developed grade 4 CTCAE. The results showed that the combination as an induction treatment is a feasible, efficacious approach for stage IIIA NSCLC.
CTONG 1103 (NCT01407822) is a phase II study of a new treatment strategy for stage IIIA-N2 NSCLC patients with EGFR activating mutations. Patients randomized to the erlotinib arm took erlotinib orally for 6 weeks in the neo-adjuvant treatment phase and for 1 year or until disease progression or unacceptable toxicity in the post-surgery phase. Patients randomized to the chemotherapy arm received GC for two cycles in each treatment phase.
CTONG 1104 (NCT01405079) is a national, multi-center, randomized, open-label, phase III trial that recruited stage II-IIIA (N1-N2) NSCLC patients with the EGFR activating mutation. The recruited patients were randomized to receive gefitinib for 24 months or vinorelbine plus platinum for four cycles after surgery. The recruiting is complete and the result will be available soon.
Trials of chemotherapy with intercalating and maintenance use of EGFR-TKIs
The EGFR mutation status of some advanced NSCLC patients cannot be determined for several reasons. The treatment strategy for such patients is worthy of investigation. The EGFR mutation rate is 49.8% in non-smokers with adenocarcinoma (15). The high EGFR mutation rate and the results of FASTACT 2 (CTONG 0902) indicated that chemotherapy with intercalating and maintenance use of EGFR-TKIs might be appropriate for selected patients. CTONG1102 enrolled never or light smokers with adenocarcinoma. It evaluated the efficacy of first-line chemotherapy (GC) with intercalating and maintenance gefitinib in selected patients with unknown EGFR mutation status. No results are yet available.
Patients with abundant common EGFR mutations might benefit most from first- line EGFR-TKIs (16). However, it is unclear how patients with uncommon mutations and few common EGFR mutations might benefit more from the approved drugs. CTONG1307 was designed for these patients. It is recruiting advanced NSCLC patients with few activating EGFR mutations. The enrolled patients are allocated to receive paclitaxel plus carboplatin and erlotinib. The uncommon EGFR mutations exclude the exon 19 deletion, exon 20 mutation, and exon 21 L858R. A low-abundant EGFR mutation means an exon 19 deletion or exon 21 L858R that is positive using real-time PCR methods and negative with standard sequencing methods. The study started recruiting patients this year.
Trials of treatment in a first-line settings
Thrombocytopenia is a common adverse effect of chemotherapy and significantly more common in patients treated with GC (17,18). CTONG1001 is recruiting patients with NSCLC who are going to receive GC/GP chemotherapy. Recombinant human thrombopoietin (rhTPO) will be given on days 2, 4, and 9 of the chemotherapy cycle or 6-24 h from the end of chemotherapy cycle to assess the efficacy and safety of administering rhTPO at different times.
Nab-paclitaxel increases the intra-tumor paclitaxel concentration. Other trials have shown that nab-paclitaxel is superior to paclitaxel (19). CTONG 1002 (NCT01236716) is a randomized phase II trial that will compare nab-paclitaxel and carboplatin with the standard first-line GC therapy in advanced squamous cell carcinoma of the lung. The primary endpoint is the overall response rate (ORR).
As an extension of CTONG 0803, CTONG 1201 is a phase III study that has enrolled EGFR-mutant NSCLC patients with brain metastasis who received no more than one line of chemotherapy to compare the efficacy of icotinib with whole-brain radiotherapy.
Trials of treatment in second/third line settings
Several retrospective studies have shown that the patients who underwent a gefitinib rechallenge might benefit more than those who did not (20,21). We designed CTONG 1304 to evaluate the DCR of gefitinib as third-line retreatment in stage IIIB/IV NSCLC patients with the EGFR 19del/L858R mutation who had benefited from first-line gefitinib treatment and had tumor progression after second-line chemotherapy.
The National Comprehensive Cancer Network (NCCN) recommended erlotinib as second-line therapy in unselected patients, although CTONG 0806 and subgroup analysis from other trials, such as FASTACT 2, had indicated that patients with wild-type EGFR would not benefit from EGFR-TKIs. CTONG 1306, a phase II trial, is designed to evaluate the efficacy and safety of erlotinib in advanced or recurrent NSCLC who failed to respond to at least one platinum-based chemotherapy regimen with wild-type EGFR and without C-met expression.
NCT01610336 is a phase IB/II, multicenter study of patients with EGFR mutated, c-MET-amplified NSCLC who have progressed after EGFR inhibitor treatment. The patients were given INC280 plus gefitinib. This is one of the few phase IB/II trials to recruit patients based on their genetic profile. This study will assess the safety and efficacy of escalating doses of INC280 in combination with gefitinib.
Observational trials
CTONG has launched three observational trials. CTONG 1202 (NCT01740804) is recruiting advanced NSCLC patients to assess the dynamic changes of circulating tumor cells (CTC) during first-line platinum-based chemotherapy, and the relationship between the CTC count and clinical outcome of chemotherapy. CTONG 1303 (NCT02042105) is recruiting all NSCLC patients to investigate the epidemiological and clinical features of ALK-positive NSCLC patients in China. CTONG 1308 (NCT02113852) was designed to collect cancer/adjacent normal tissue and a matched blood sample from NSCLC patients who are heavy smokers to identify and characterize the features of the genome and transcriptome in NSCLC patients.
Most of these ongoing trials are intended to extend the indications for EGFR-TKIs, so that more patients can benefit fully from these effective drugs. The results of most of the ongoing trials mentioned above are pending. We anticipate that these results will contribute to changing clinical practice.
Future clinical trials
The ideal option is to combat tumors with effective antitumor drugs that have low toxicity, such as gefitinib and erlotinib. Compared with chemotherapy, targeted drugs significantly improve patient quality of life and prolong survival. At present, however, only icotinib, gefitinib, erlotinib, and crizotinib have been approved by the State Food and Drug Administration (SFDA). Consequently, only 35% of NSCLC and 0% of small-cell lung cancer (SCLC) can benefit from targeted therapy at present (15). There is an urgent need to identify novel therapeutic targets and develop effective drugs for them.
We plan to launch a phase II cluster study of advanced NSCLC patients in China (22). These patients will be allocated to a specific treatment arm based on their genetic profile: AUY922 for patients with an activating EGFR mutation who are resistant to EGFR inhibitors; BYL719 for patients with a PIK3CA mutation/amplification (other PI3K pathway alterations may be eligible); INC280 for patients with MET-positive tumors [by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH)]; LDK378 for patients ALK or ROS1 rearrangement; and MEK162 for patients with KRAS, NRAS, or BRAF mutations. Each treatment arm is independent of the others and will be analyzed separately.
As the lung cancer genomic landscape is revealed, more targetable oncogenic drivers with a low incidence will be discovered. Launching separate trials examining each oncogenic driver and the corresponding drugs would be too time consuming. Consequently, clustering of clinical trials will likely become a trend in the near future.
Conclusions
The majority of the completed, ongoing, and planned trials involve drugs that are a milestone in the treatment of lung cancer. The completed trials have established the position of EGFR-TKIs in the treatment of Chinese patients. The ongoing trials will extend the indications for EGFR-TKIs. We hope that Chinese investigators will lead more international trials in the future and contribute more to lung cancer patients worldwide.
Acknowledgements
Grant support: This work was supported by grants from the National Natural Science Foundation of China (81001031, 81372285), grant S2013010016354 from the Natural Science Foundation of Guangdong.
Disclosure: The authors declare no conflict of interest.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- Zhou C, Chen G, Liu X, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicentre, phase III study of first-line carboplatin/paclitaxel (CP) plus bevacizumab (Bv) or placebo (Pl) in Chinese patients with advanced or recurrent non-squamous non-small cell lung cancer. J Thorac Oncol 2013: (suppl; abstr MO06.13). Available online: http://abstracts.webges.com/myitinerary/publication-2756.html?congress=wclc2013
- Wu YL, Lu S, Cheng Y, et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 2014;85:401-7. [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [PubMed]
- Sun Y, Wu YL, Zhou CC, et al. Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer 2013;79:143-50. [PubMed]
- Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol 2013;24:993-9. [PubMed]
- Yang J, Cheng Y, Zhao M, et al. A phase II trial comparing pemetrexed with gefitinib as the second-line treatment of nonsquamous NSCLC patients with wild-type EGFR (CTONG0806). J Clin Oncol 2013;31:abstr 8042.
- Goss GD, Lorimer I, Tsao MS, et al. A phase III randomized, double-blind, placebo-controlled trial of the epidermal growth factor receptor inhibitor gefitinb in completely resected stage IB-IIIA non-small cell lung cancer (NSCLC): NCIC CTG BR.19. J Clin Oncol 2010;28:abstr LBA7005.
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:abstr 7514.
- Shepherd FA, Altorki NK, Eberhardt WE, et al. Poster Highlights Session, Lung Cancer - Non-small Cell Local-regional/Small Cell/Other Thoracic Cancers. J Clin Oncol 2014;32:abstr 7513. Available online: http://meetinglibrary.asco. org/content/130009-144
- Lu S, Jiang G, Chen Z, et al. A single arm, multi-center, phase II study of intercalated erlotinib with gemcitabine/cisplatin as neoadjuvant treatment in stage IIIA non-small cell lung cancer (CTONG 1101, NCT01297101): preliminary result. J Thorac Oncol 2013: (suppl; abstr P1.09-016 ). Available online: http://abstracts.webges.com/myitinerary/publication-2041.html?congress=wclc2013
- An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One 2012;7:e40109. [PubMed]
- Zhou Q, Zhang XC, Chen ZH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:3316-21. [PubMed]
- Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-91. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794-803. [PubMed]
- Nishino K, Imamura F, Morita S, et al. A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer 2013;82:299-304. [PubMed]
- Watanabe S, Tanaka J, Ota T, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer 2011;11:1. [PubMed]
- Zhou Q, Zhang XC, Peng B, et al. A phase II cluster study of single agent AUY922, BYL719, INC280, LDK378, and MEK162 in Chinese patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr TPS8122.


