
Flexible bronchoscopy-guided microwave ablation in peripheral porcine lung: a new minimally-invasive ablation
Introduction
Lung cancer is the leading cause of cancer death in the world (1). Surgical resection is the standard cure for patients with stage I or II non-small-cell lung cancer (NSCLC). However, a significant number of patients with NSCLC are not candidates for surgery due to severe medical complications, primarily associated with deteriorated lung function (2). Minimal invasive therapies including percutaneous image-guided ablation and stereotactic body radiation therapy (SBRT) have been developed to give an option for curative treatment in early stage patients (3). However, percutaneous approaches can develop difficult complications [pneumothorax (PTX), fistula] and SBRT can induce radiation pneumonitis. In this poor functioning group, complications like this affect quality of life and show modest improvement in mortality (4).
Image-guided minimal invasive ablative therapies are delivered by using needlelike applicators including high-temperature ablation (such as radiofrequency, microwave and laser), chemical ablation, cryoablation and irreversible electroporation (5-7). Radiofrequency ablation (RFA) and microwave ablation (MWA) have larger evidence and experience. MWA has several theoretical advantages over RFA in producing higher temperatures and larger volumes, less ablation time, less dependence on the electrical conductivities of tissue, and is less limited by the rising electrical impedance of tumor tissue (8-10). MWA functions by destroying tumor through the application of high temperature generated by electromagnetic energy to rapidly rotate adjacent polar water molecules within the targeted pathologic tissue, which leads to protein denaturation, cell membrane disruption, and finally coagulation necrosis with cellular death (10). These changes appear within 4–6 minutes of treatment at a temperature greater than 50 °C and occur almost immediately at 60 °C (8,11). Percutaneous use of MWA has been widely used in liver cancer therapy (12), and has become the preferred energy modality in the percutaneous ablation of air-filled pulmonary neoplasms, particularly for big lesions or those close to large vessels which may cause rapid dissipation of the applied heat via “heart sinks” (8,13,14). Also internally cooled applicator had been used to cause larger coagulation necrosis and avoid unnecessary damage to the surrounding tissue (11). Lung ablation therapy is commonly performed percutaneously under image-guidance from computed tomographic (CT) or ultrasound. The incidence of PTX (occurring in 15% of patients, 6% of which require chest tube placement) is similar to that of percutaneous lung biopsy, indicating no relation to thermal ablation itself (15,16). With the development of several guided-bronchoscopy technologies, the procedural risk for PTX is lower than that under CT image-guidance (occurring in <2% of patient, <1% of which required chest tube placement) (17). A new approach that combines the high efficiency of MWA and a favorable safety profile of bronchoscopy is needed.
Bronchoscopy-guided, internally water-cooled MWA utilizes a new flexible microwave antenna which fits through the working channel of a therapeutic bronchoscope with enough length to reach the lung lesion invading the natural bronchial lumen. There used to be a flexible bronchoscopically-guided, gas (CO2)-cooled MWA antenna (Neuwave, Madison, WI, USA) (18). The difference was the cooling method, water-cooling was readily available and stored in the clinic while gas-cooling had a higher cooling efficiency.
In this study, we describe a new approach of lung ablation using a flexible water-cooled MWA under bronchoscopy and evaluate its feasibility and safety in both ex vivo and in vivo animal models (lung).
Methods
The study protocol was approved by the Ethics Committee at Shanghai Chest Hospital (Shanghai, China) (KS1610).
All studies were performed by using the microwave platform (KY-2000, Canyon Medical Inc., Nanjing, China). The microwave unit consists of a 2.45 GHz generator, a flexible coaxial cable, a water-pumping machine and a flexible bronchoscopy-guided, internally water-cooled MWA antenna (Canyon Medical Inc, Patent 201620582789.0) with a diameter of 1.90 mm and a length of 1,150 mm, and a 7 mm long stainless tip (Figure 1A, 1). Inside the cooled-catheter, there are dual channels in which 4 °C physiological saline water is continuously extracted, circulated and discharged with 42 mL per minute flow rate. The temperature of the catheter is monitored through a thermocouple measuring the circulation water pumped-out in real time. To simulate in vivo conditions, all ex vivo tissues were perfused with 37 °C saline water.
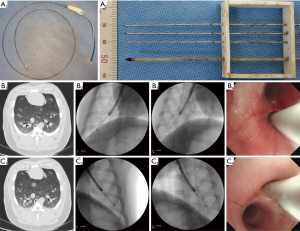
Ablation by antenna penetrated through the surface of normal ex vivo lung
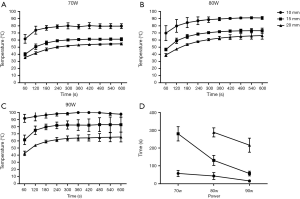
In study 1, five fresh ex vivo porcine lungs were collected and uninflated. The antenna was directly penetrated at least 5 cm from the pleural surface. Ablations were set at 70, 80, 90 W for 10 minutes respectively. A total of 9 ablations were performed (three repetitions per power). Temperatures of three sites (10, 15, and 20 mm peripheral to the heating zone) were measured by thermocouples parallel away from the antenna tip with 5 mm intervals (Figure 1A, 2). Temperature-time curves were calculated and the total time of temperature up to 60 °C at site 20 mm were evaluated.
Ablation by antenna through the working channel of bronchoscopy in ex vivo and in vivo lung
In study 2, three fresh ex vivo porcine lungs were collected and inflated under the control of ventilator (SAVINA, Dräger, Germany) with a tracheal intubation. Synchronized intermittent mandatory ventilation (SIMV) mode, tidal volume (VT) 500 mL, inspiratory time (Tinsp) 1.6 seconds (s), frequency (F) 14 breath per minute (bpm), inspiratory pressure (Pinsp) 14 mBar, positive end-expiratory pressure (PEEP) 4 mBar were set. The flexible MWA antenna covered by a protection tube [extended working channel (EWC), inReach system, Covidien, America] safely pass the working channel of a flexible video-bronchoscope (model 1T-260; biopsy channel diameter 2.8 mm; Olympus; Tokyo, Japan). This set of apparatus was accurately located to the second dorsolateral branch of the septal bronchus in bilateral lung. The optimal parameter, 80 W for 5 minutes was used. A total of 6 ablations were performed with three repetitions per lobe. Lesion Dl, Ds were measured and Volume (Vol = 1/6 × π × Dl × Ds2) was calculated (19).
In study 3, six pigs weighing 60–65 kg each were sedated with 200 mg of Xylazine Hydrochloride (Huamu Animal Medical Inc., Jilin, China) intramuscularly and anesthesia was maintained with 4–6 mg/kg/hour of propofol (Hospira Inc., Lake Forest, USA) intravenously after fasting overnight. All animals were scanned by CT (Philips Brilliance 64 multi-slice spiral CT, Philips Healthcare, The Netherlands) with 0.67-mm-thick contiguous transverse computed tomographic sections. Virtual bronchoscopic navigation (VBN; DirectPath; Olympus, Tokyo, Japan) were used for assistance in navigation. Tracheal intubation was inserted and breathing under the control of ventilator (SAVINA, Dräger). The flexible MWA antenna covered by EWC, was guided to the target bronchus through the working channel of the bronchoscope (1T-260, Olympus). The procedure was fluoroscopically-guided by the C-arm (Philips Veradius Unity, Philips Healthcare, The Netherlands). The positions of ablation were located to the second dorsolateral branch of the septal bronchus on CT and the orientation of the tip was adjusted towards the target, at least 10mm away from pleura confirmed with multiple fluoroscopy projections in right (Figure 1B) and left (Figure 1C) lung, representatively shown. The ablation parameter, 80 W for 5 minutes, was set. Continuously electrocardiography and pulse oximetry were monitoring. Twelve total ablations were performed. After the operation, a CT scan was performed immediately. All ablations were divided into groups based on sacrifice time (group A, 24 hours, n=6; group B, 4 weeks, n=6). Additional CT scan and bronchoscopy were performed at intervals of 24 hours (12/12 ablations), 2 weeks (6/12 ablations), 4 weeks (6/12 ablations). Two maximal dimensions in both axial and coronal plane were measured for lesions of CT images by using ruler, as previously described (19,20). The ablation zones dissected were along the axis of bronchus where the antenna inserted and Dl, Ds were measured. Each vivo ablated zone was then stored in 10% formalin for paraffin sectioning, hematoxylin-eosin (HE) staining, Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) staining assay, Masson trichrome staining for microscopic study were performed by pathologists, as previously described (21,22).
Statistical analysis
Results were presented as mean ± SD and analyzed using SPSS version 20.0 statistical software (IBM, New York, United States). Comparisons between groups were performed by t-test. A P value <0.05 was considered significant.
Results
In study 1, temperature of site 10 mm, 15 mm at 70, 80, 90 W could reach the effective temperature needed for ablation. At site 20 mm only the power settings of 80 and 90 W could touch the effective temperature and the active time of 80 W was 288±26 s, 90 W was 216±39 s (Figure 2).
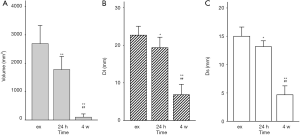
In study 2 and 3, all ablation lesions (n=18, 6 in ex vivo and 12 in vivo) dissected from the porcine lung could be observed macroscopically. In ex vivo lung, the mean Dl was 22.7±2.3 mm and mean Ds was 15.0±1.5 mm. In vivo, the mean Dl of 24 hours was 19.3±2.7 mm and mean Ds of 24 hours was 13.2±1.0 mm, while the mean Dl of 4 weeks was 6.8±2.6 mm and mean Ds of 4 weeks was 4.7±1.5 mm. The length of the gross observation varies in ex vivo and in vivo at different time (P<0.01) (Figure 3).
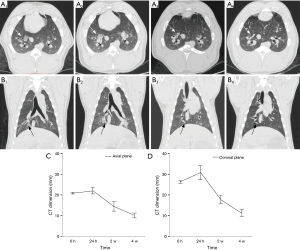
No major complications (e.g., PTX and pulmonary parenchymal hemorrhage) occurred during the procedure and the post-procedural period. Common CT findings after immediate ablation were areas of ground glass opacity (GGO) around the area of antenna activity. After an initial increase in 24 hours, there was a persistent reduction in diameter of the ablated areas at subsequent examinations, consistent with consolidation of the pulmonary parenchyma. Most lesions were nodular at 4 weeks on CT (Figure 4).
Histopathology assessment
Twenty-four hours after ablation (group A)
The ablation zone was centered along the bronchus at the area of antenna insertion. Under HE staining, a succession of three concentric layers with different characteristics were seen around the bronchus. The bronchial mucosa (BM) and cartilage still retained the original organization structure. The inner layer show signs of thermal damage but the tissue maintains a seemingly intact alveolar structure with little effusion. Using a TUNEL Assay, the epithelium of BM was almost completely apoptotic, but bronchial cartilage was still viable without any apoptosis. The inner layer of ablation center suggested that cells were almost completely apoptotic (Figure 5).
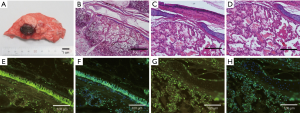
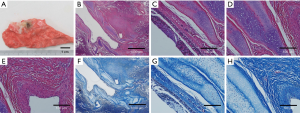
Four weeks after ablation (group B)
A circle of fibrous scar tissue was formed around the bronchus. The BM and cartilage were still intact and plenty of fibrous tissue could be seen in the ablation center. Using a Masson trichrome staining, the fibroblast cells or tissues underneath BM were seen, while the focal area of ablation is completely replaced by the fibroblast cells or tissues (Figure 6).
Discussion
During years prior, various percutaneous image-guided techniques for ablation have been developed for the treatment of lung tumors. The risk of complications was related to the increased length of the lung traversed, similar to percutaneous lung biopsy (15,23). The goal of using natural channels to reach the lesions and provide energy ablation safely has been demonstrated in the previous studies (24,25). Tsushima et al. (26) showed that, bronchoscopy-guided RFA was safe and feasible in sheep lungs. Xie et al. (27) reported navigation bronchoscopy-guided RFA for nonsurgical peripheral pulmonary tumors with success and a good safety profile.
There are three criteria to assign malignant nodules in lung detected on CT images: solid noncalcified nodules, pure ground-glass nodule and part-solid lung nodules (28). The component of ground-glass nodule on CT corresponds pathologically to alveolar septal thickness and contained air in the alveoli, namely lepidic growth preserving the existing alveolar structure of the cancer cells (29). As RFA is dependent on thermal conductivity, air-filled tumors tend to limit the size of the ablation zone. Microwave propagation is thought not to be similarly hindered by air and could be more easily applied to various types of tumors (30).
Flexible bronchoscopy-guided, water-cooled MWA is a relatively new technique that can avoid the risk of percutaneous operation and not be limited to air in the tumor. This is the first report showing the feasibility and safety of the flexible bronchoscopy-guided, water-cooled MWA in ex vivo and in vivo porcine lung.
The goals of our thermal ablation study were a 5–10 mm safety margin of apparently healthy tissue adjacent to tumor, avoiding injury to critical structures, and create a large ablation area quickly (5,11,31). From prior work, it appears that thermal ablation works well, particularly in local control, for early-stage or small tumors (<30 mm) (32). In light of this, we originally set the range of ablation to the 20 mm radius with a 5 mm safety margin. In the temperature-rising curve of ex vivo lung, 80 W for 5 min extended the coagulation necrosis temperature to the 20 mm radius. During the in vivo live model, using the same 80 W for 5 minutes, the range was decreased sharply far from 20 mm radius. Respiratory motion and vascular factor (“heat-sink” caused by pulmonary blood flow) were likely a leading cause.
No serious complications occurred. CT imaging characteristics of the ablation zone were consistent with a GGO immediately after ablation illustrating the effectiveness of ablation, similar to percutaneous ablation (33). The lesion gradually changed into nodules with persistent reduction in size.
The limitations of previous clinical RFA studies done near the trachea are theoretically influenced by the trachea wall thickness (34). Our pathology at 24 hours also really found that there was no apoptosis in bronchus cartilage, which possibly affect the energy deposition of ablation. But electromagnetic energy field of MWA could still cause coagulation necrosis, similar to percutaneous ablation (35), in lung parenchyma around bronchus cartilage. Compared with RFA, the advantage of MWA is a demonstrated greater ablation range. Pathology at 4 weeks showed that the intact preservation of bronchus cartilage was helpful to repair the ablation area and reduce the production of unnecessary inflammation, especially obstructive inflammation.
This study has several limitations. First, our animal model was using normal healthy tissue lacking a tumor model. The properties of lung tumors are different from the properties of normal lung parenchyma. However, our experiment made it clear that MWA could produce ablation effect in air-filled normal lung tissue, which could mimic air-filled ground glass pulmonary neoplasms (36), the main objective of MWA. Second, although no major complications were observed during this study, the number of animal subjects and ablations done limit any ability to absolutely comment on safety. Third, the range of ablations under bronchoscopy were far from ideal percutaneous and more attempts need to be made and improved. Artificial atelectasis (e.g., target bronchial balloon catheter block) and ventilation reduction strategy can be tried. Fourth, the real-time temperature measurement and ablation range were not real-time monitor during the endobronchial operation. We propose a preliminary solution that setting real-time temperature measuring points on the catheter different distance from the tip of antenna. In summary, we have successfully used a flexible bronchoscopy-guided microwave antenna to perform ablation in the lung via the natural bronchial lumen in an animal and it appears to be effective and safe.
Acknowledgments
Funding: This work was supported by The National Key R&D Program of China (2017YFC0112700); Clinical Research Plan of SHDC (16CR3007A); Scientific Research Program by Science and Technology Commission of Shanghai Municipality (16441900702, 15411964300).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee at Shanghai Chest Hospital (Shanghai, China) (KS1610).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage copd. Int J Chron Obstruct Pulmon Dis 2016;11:1317-26. [Crossref] [PubMed]
- Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res 2017;6:131-47. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage i non-small cell lung cancer: A meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [Crossref] [PubMed]
- Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J 2016;34:160-7. [Crossref] [PubMed]
- Yuan Z, Wang Y, He ML, et al. Successful ablation for pulmonary artery tumor thrombosis more than 5 cm with massive hepatocellular carcinoma and multiple pulmonary metastases. Transl Cancer Res 2018;7:456-61. [Crossref]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-9. [Crossref] [PubMed]
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur J Radiol 2011;79:124-30. [Crossref] [PubMed]
- Goldberg SN, Gazelle G, Mueller P. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31. [Crossref] [PubMed]
- Ierardi AM, Giorlando F, Piacentino F, et al. Factors predicting outcomes of microwave ablation of small hepatocellular carcinoma. Radiol Med 2017;122:81-7. [Crossref] [PubMed]
- Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (nsclc) in the elderly: A promising outlook. J Med Imaging Radiat Oncol 2015;59:82-90. [Crossref] [PubMed]
- Carberry GA, Nocerino E, Mason PJ, et al. Pulmonary microwave ablation near the heart: Antenna positioning can mitigate cardiac complications in a porcine model. Radiology 2017;282:892-902. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Lehnert T, et al. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations--review article. Eur J Radiol 2011;77:346-57. [Crossref] [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: An analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Ferguson J, Egressy K, Schefelker R, et al. Bronchoscopically-Guided Microwave Ablation in the Lung. Chest 2013;144:87A. [Crossref]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Ridge CA, Huang J, Cardoza S, et al. Comparison of multiplanar reformatted ct lung tumor measurements to axial tumor measurement alone: Impact on maximal tumor dimension and t stage. AJR Am J Roentgenol 2013;201:959-63. [Crossref] [PubMed]
- Rychel AL, Swalla BJ. Anterior regeneration in the hemichordate ptychodera flava. Dev Dyn 2008;237:3222-32. [Crossref] [PubMed]
- Yamamoto M, Murata K, Kiriu T, et al. Acute fibrinous and organizing pneumonia with myelodysplastic syndrome: Corticosteroid monotherapy led to successful ventilator weaning. Intern Med 2016;55:3155-9. [Crossref] [PubMed]
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: Incidence and risk factors. Radiology 2006;241:275-83. [Crossref] [PubMed]
- Krimsky WS, Pritchett MA, Lau KKW. Towards an optimization of bronchoscopic approaches to the diagnosis and treatment of the pulmonary nodules: A review. J Thorac Dis 2018;10:S1637-44. [Crossref] [PubMed]
- Vieira T, Stern JB, Girard P, et al. Endobronchial treatment of peripheral tumors: Ongoing development and perspectives. J Thorac Dis 2018;10:S1163-7. [Crossref] [PubMed]
- Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J 2007;29:1193-200. [Crossref] [PubMed]
- Xie F, Zheng X, Xiao B, et al. Navigation bronchoscopy-guided radiofrequency ablation for nonsurgical peripheral pulmonary tumors. Respiration 2017;94:293-8. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on ct images: From the fleischner society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Aokage K, Miyoshi T, Ishii G, et al. Influence of ground glass opacity and the corresponding pathological findings on survival in patients with clinical stage i non-small cell lung cancer. J Thorac Oncol 2018;13:533-42. [Crossref] [PubMed]
- Pua BB, Thornton RH, Solomon SB. Ablation of pulmonary malignancy: Current status. J Vasc Interv Radiol 2010;21:049.31.
- Sidoff L, Dupuy DE. Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia 2017;33:25-33. [Crossref] [PubMed]
- Klapper JA, Hittinger SA, Denlinger CE. Alternatives to lobectomy for high-risk patients with early-stage non-small cell lung cancer. Ann Thorac Surg 2017;103:1330-9. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: Effectiveness, ct findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-guided cooled radiofrequency ablation as a novel intervention therapy for peripheral lung cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency ablation in a porcine lung model: Correlation between ct and histopathologic findings. AJR Am J Roentgenol 2005;185:1299-306. [Crossref] [PubMed]
- Koo CW, Miller WT, Kucharczuk JC. Focal ground-glass opacities in non-small cell lung carcinoma resection patients. Eur J Radiol 2012;81:139-45. [Crossref] [PubMed]


