
Combination immune checkpoint inhibitors with platinum-based chemotherapy in advanced non-small cell lung cancer: what’s known and what’s next
Immune checkpoint inhibitors (ICIs) that target programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have revolutionized the treatment paradigm for patients with advanced or metastatic non-small cell lung cancer (NSCLC) (1). Yet, only a minority of patients could derive clinical benefit from anti-PD-1/PD-L1 antibody monotherapy (2). Both scientists and physicians aimed to enhance the therapeutic efficacy and expand benefit populations via investigating the rational combination therapeutic strategies and predictive biomarkers (2-6). Since chemotherapy could elicit anticancer immunity via release of potentially immunogenic tumor antigens (7,8), combination of anti-PD-1/PD-L1 antibodies with chemotherapy is one of the most anticipated strategy in this field.
In The Lancet Oncology, West and colleagues reported the results of IMpower130, a multicentre, randomized, open-label, phase III placebo-controlled trial, investigating atezolizumab (anti-PD-L1 antibody) in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous NSCLC (9). The study met its co-primary end-points, since atezolizumab plus chemotherapy led to a significant improvement in overall survival (OS) and progression-free survival (PFS) as compared to chemotherapy. In the intention to treat wild-type population, the median OS was 18.6 vs. 13.9 months [hazard ratio (HR): 0.79; 95% confidence interval (CI): 0.64–0.98; P=0.033], and the median PFS was 7.0 vs. 5.5 months (HR: 0.64; 95% CI: 0.54–0.77; P<0.0001). The addition of atezolizumab to platinum-based chemotherapy resulted also in a significant increase of 1-year survival rate (63.1% vs. 55.5%), 2-year survival rate (39.6% vs. 30.0%), objective response rate (ORR) (49.2% vs. 31.9%), and duration of response (8.4 vs. 6.1 months). The survival benefit was maintained in all subgroups of patients selected according to both clinical and pathological characteristics, except for patients with liver metastases and those with EGFR or ALK genomic alterations. Notably, treatment benefit was observed in terms of OS and PFS in the intention-to-treat wild-type populations, regardless of PD-L1 expression. Unsurprisingly, the number of patients who experienced severe adverse events (SAEs) and was significantly higher with immune-chemotherapy combination than chemotherapy alone (51% vs. 38%), with treatment-related SAEs reported to be 24% vs. 13%, respectively. The percentage of Immune-related adverse events was also significantly higher in combination group (45%). Finally, the percentage of treatment-related (any treatment) deaths nearly doubled with atezolizumab plus chemotherapy compared to chemotherapy group.
Although more than 60% of patients in the chemotherapy group received at least one subsequent line of immunotherapy when disease progression occurred, IMpower130 still showed the OS benefit in combination group. Furthermore, OS and PFS benefits were observed in the majority of demographic subgroups. Interestingly, this combination therapy did not improve OS and PFS in patients with liver metastases compared with the chemotherapy alone group, which is a noticeable and novel observation. Initial publications of KEYNOTE-407 and KEYNOTE-189 did not show the subgroup data on the basis of the presence of liver metastases (10,11). The IMpower150 study showed prolonged PFS in patients with liver metastases received atezolizumab plus bevacizumab plus carboplatin and paclitaxel rather than bevacizumab plus carboplatin and paclitaxel (12). Further investigations of the mutational and immune landscape of primary lesions and liver metastases might be worthwhile to unravel the potential mechanism. Similarly, atezolizumab plus carboplatin plus nab-paclitaxel did not show survival benefit in patients with EGFR or ALK genomic alterations while atezolizumab plus bevacizumab plus carboplatin and paclitaxel could result in the improved outcomes (12). These results were reminiscent of our current finding that addition of bevacizumab might synergize with PD-1/PD-L1 inhibition (13). Additionally, IMpower130 did not detect a significant difference among subgroups with different PD-L1 expression level. Inconsistently, KEYNOTE-189 study reported that survival benefit seemed to be associated with PD-L1 tumor proportion score (TPS) and the greatest survival benefit was observed in the subgroup with PD-L1 TPS ≥50% (11). In the future, we still need more clinical data to clarify the predictive value of PD-L1 expression in predicting the efficacy of immune-chemotherapy combination.
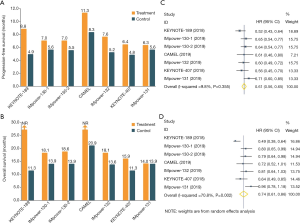
Several landmark phase III trials have reported that combination of anti-PD1/PD-L1 antibodies and chemotherapy showed the increased antitumor efficacy in both advanced squamous and non-squamous NSCLC (Figure 1) (9-12,14-16). These studies consistently demonstrated that anti-PD1/PD-L1 antibodies plus chemotherapy could provide the significant improvement in both PFS (Figure 1A) and OS (Figure 1B), except for IMpower131 (atezolizumab plus chemotherapy did not prolong OS in lung squamous cell carcinoma). Moreover, when we conducted a meta-analysis of these trials by including 4,250 cases, we also found that anti-PD1/PD-L1 antibodies in combination with chemotherapy could result in the substantially prolonged PFS (HR: 0.61, 95% CI: 0.56–0.65; P<0.0001; I2=9.8%; Figure 1C) and OS (HR: 0.74, 95% CI: 0.61–0.86; P=0.002; I2=70.8%; Figure 1D). Collectively, these findings further strengthen the primary recommendation of immune-chemotherapy combination for patients with advanced or metastatic NSCLC.
Despite of the remarkable antitumor efficacy of immune-chemotherapy combination, treatment resistance was eventually inevitable. Up to now, there was very few studies to report the possible resistance mechanism. The majority of patients would have limited therapeutic option after disease progression. Therefore, we need to explore the potential resistance mechanism to immune-chemotherapy combination, which would lay a foundation for the development of novel strategies to overcome treatment resistance and prolong survival benefit. It is very useful to carry out the relevant translation research by using the tissue and blood samples from these clinical trials. In addition, immune-chemotherapy combination still cannot benefit all patients with NSCLC. We still need to investigate the predictive biomarkers. As we mentioned before, PD-L1 expression level was not correlated with the efficacy of atezolizumab plus chemotherapy. Recent study reported that there was no association between tumor mutation burden (TMB) and outcomes in both immune-chemotherapy and chemotherapy alone group (KEYNOTE-189 study). It may be not enough to consider tumor itself when we study the predictive biomarkers for immune-chemotherapy combination. We should take the immune microenvironment and host immunity into consideration in the future investigations.
In summary, the study of West et al. (9) provides the additional data in favor of the use of first-line anti-PD-1/PD-L1 antibodies plus chemotherapy in advanced or metastatic non-squamous NSCLC. Atezolizumab in combination with carboplatin plus nab-paclitaxel demonstrated a significant and clinically meaningful improvement in both OS and PFS, together with an acceptable toxicity, in patients with stage IV non-squamous NSCLC without EGFR and ALK alterations, offering another treatment option for these populations. Long-lasting follow-up of this study will be critical to establish the long-term efficacy and tolerability outcomes and definitively confirm first-line immunotherapy plus chemotherapy as the right strategy to fight non-squamous NSCLC. In addition, we still need to explore the novel biomarkers of immune-chemotherapy combination to further enhance the therapeutic benefit and strategies to overcome combination treatment resistance.
Acknowledgments
Funding: This study was supported in part by grants from the National Natural Science Foundation of China (No. 81672286, 81772467 and 81874036).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Low JL, Walsh RJ, Ang Y, et al. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Ther Adv Med Oncol 2019;11:1758835919870360. [Crossref] [PubMed]
- Arora S, Velichinskii R, Lesh RW, et al. Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv Ther 2019;36:2638-78. [Crossref] [PubMed]
- Galluzzi L, Chan TA, Kroemer G, et al. The hallmarks of successful anticancer immunotherapy. Sci Transl Med 2018. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018;48:417-33. [Crossref] [PubMed]
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Jiang T, Shi T, Zhang H, et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol 2019;12:93. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [PubMed]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000. [Crossref]
- Papadimitrakopoulou V, Cobo M, Bordoni R, et al. IMpower132: PFS and Safety Results with 1L Atezolizumab + Carboplatin/Cisplatin + Pemetrexed in Stage IV Non-Squamous NSCLC. J Thorac Oncol 2018;13:S332-3. [Crossref]
- Zhou C, Chen G, Huang Y, et al. A Randomized Phase 3 Study of Camrelizumab plus Chemotherapy as 1st Line Therapy for Advanced/Metastatic Non-Squamous Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:S215-6. [Crossref]


