
Toxicity of tumor immune checkpoint inhibitors—more attention should be paid
Introduction
Tumor immunotherapy can be grouped into two categories: active immunotherapy and passive immunotherapy. Active immunotherapy acts by stimulating and enhancing the host’s antitumor immune response, which is also divided into specific and non-specific, the former using tumor-specific antigen, the latter using non-specific substances which can stimulate the immune system. Several ICIs have been used in cancer therapy (Table 1). Programmed death receptor-1/ligand-1 (PD-1/L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) checkpoint inhibitors are the most widely used. ICIs can activate non-specific immunity and cause irAEs. The pathophysiological mechanisms have not been fully elucidated, but are currently thought to be related to the invasion of normal tissue by immune cells. This paper mainly discusses the toxicities of ICIs in anti-tumors treatment.
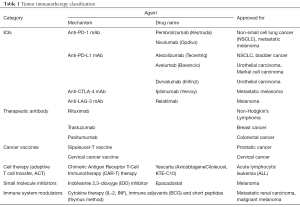
Full table
Dermatologic toxicities
Anti-PD-1/PD-L1 agents could lead to dermatologic toxicity (1). It has been reported that skin toxicity may occur in more than 40% melanoma patients treated with anti-PD-1 monoclonal antibodies (mAbs) (2). A meta-analysis showed that the average incidence of dermatotoxicity is 44% in patients receiving anti-CTLA-4 therapy (3). The median times of onset are in the range of 4–8 weeks for nivolumab, 23 weeks for pembrolizumab (4) and 4–10 weeks for ipilimumab (5). Most of the clinical manifestations are skin rash with or without skin itching (usually mild to moderate), and the typical affected parts are trunk and limbs, which can be effectively controlled by timely treatment. In addition, PD-1 inhibitors have mucosal toxicity, which is manifested as dry mouth and mucositis. Patients treated with ICIs also have a very low risk of lethal Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) (6). Histologically, there are usually signs of spongiotic or lichenoid dermatitis features when performed the biopsy, and we also find the perivascular infiltration is rich in T lymphocytes (7).
The severity of the following diseases is classified according to the Common Terminology Criteria for Adverse Events (CTCAE) to guide treatment. ICIs can continue to be used for grade 1 skin adverse events such as rashes and/or itching. Topical emollients, oral antihistamines and/or topical weakly potent corticosteroids are used. With effective treatment, ICI induced rashes resolve almost completely within 1–2 months, although some patients have persistent and/or recurrent low-level skin toxicity after completion of subsequent treatment (8). Patients with grade 2 adverse skin events can continue to use ICIs, but need to check weekly for improvement in adverse skin events. If there is no improvement, stop the ICIs until the adverse skin events are reduced to level 1. When facing level 3 adverse skin event, the use of ICIs will be stopped immediately until it reduced to level 1. According to the severity of symptoms, systemic use of corticosteroids 0.5–1 mg/kg can also be considered. Grade 4 cutaneous toxicity is rare, if so, ICIs should be discontinued, and the patient should be admitted to the hospital as soon as possible for treatment with the help of dermatologist. Treatment includes intravenous (methylated) prednisolone 1 to 2 mg/kg, which is then gradually reduced as the toxicity subside (5).
Digestive toxicities
Gastrointestinal toxicities
Gastrointestinal mucosa can absorb nutrients and resist the invasion of foreign pathogens, because it is at the forefront of foreign material contact, it’s also the focus on immunotherapy side effects. CTLA-4 plays a pivotal role in the maintenance of intestinal immune homeostasis (9). In this homeostasis, CD4+CD25+Treg can mediate tolerance to autoantigens and also impede host protective immune responses to tumors and pathogens. Anti-CTLA-4 mAbs treatment may affect Treg-mediated suppression of T cell responses and alter the balance of intestinal immune regulation, usually leading to enterocolitis (10). In addition, immunotherapy also affect the genetic and microbiota resulting in gastrointestinal toxicity (9). Gastrointestinal toxicity is observed in about 35% of patients in anti-CTLA-4 mAbs dose-dependent manner (11). However, the incidence of gastrointestinal toxicity in anti-PD-1 mAbs is relatively reduced 6.0–16.0% (12). Gastrointestinal toxicities’ median time to onset is in the range of 6 weeks for nivolumab, with a considerably longer median (18 weeks) reported for colitis of any grade for pembrolizumab (4) and 5–10 weeks for ipilimumab (5). Immune-mediated gastrointestinal toxicity initially manifests as diarrhea, nausea, bloody stools, electrolyte disturbance, vomiting, or abdominal pain, leading to intestinal obstruction and intestinal perforation. Compared with sigmoid colon, ascending colon and rectum, irAEs’ colitis is more likely to occur in the descending colon (13). Computed tomography (CT) images of the abdomen show edema and thickening changes in the colon wall, colonoscopy is still the gold standard for confirming immune-associated colitis. Normal mucous membranes, mild erythema with mucous granules and/or severe ulcer can be seen at colonoscopy (14).
Patients who do not have severe diarrhea should be treated with antidiarrheal rehydration and electrolyte replacement if necessary and ICIs could be continued. Patients with persistent grade 2 diarrhea or severe diarrhea should stop ICIs and receive systemic glucocorticoid therapy (intravenous infusion of 1–2 mg/kg per day). For those who are effective with intravenous glucocorticoid within 3–5 days, we can convert to oral glucocorticoid therapy, and if not effective within 8–12 weeks we should convert to infliximab therapy under no contraindications. In general, single dose of infliximab (5 mg/kg) is sufficient (15). Studies have suggested that vedolizumab can replace infliximab (16).
Hepatic toxicities
The incidence of hepatic toxicity is relatively low. Among the melanoma patients treated with ICIs, the incidence of hepatic toxicity is about 1–2% (17). In contrast, the incidence of hepatic toxicity is significantly increased with combination therapy, about 15%, among which more than 50% are grade 3–4 immune-mediated hepatic toxicity (18). Median time to onset is highly variable with 25 weeks (range, 4–31 weeks) in lung cancer patients under anti-PD-1 treatment (4) and 6 weeks under anti-CTLA-4 agents (5). In general, immune-mediated hepatotoxicity is usually with elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Increased total bilirubin may be associated with jaundice and fatigue. Severe immune-mediated hepatotoxicity may even result in clinical death. Hepatotoxicity of immunosuppressive agents show non-specific imaging manifestations, but other etiologies (lymphadenopathy, gallbladder edema, periportal edema, steatosis) could be excluded by imaging examination (19). During liver dysfunction, biopsy can be conclusive, and histopathologic findings are usually lymphocytic infiltration. Histiocytic aggregates and confluent necrosis are rare in anti-PD-1/PD-L1 therapy, but are common in anti-CTLA-4 therapy (20).
Patients with moderate (grade 2) elevation of transaminase or total bilirubin should discontinue ICIs and have serum transaminase and bilirubin levels tested twice a week. If the level 2 is elevated for more than 1 to 2 weeks, corticosteroid therapy with a dose of 1 mg/kg/d (methyl) prednisolone or other equivalent drugs is required after other pathogenic factors are excluded. Once improved, ICIs can be continued after corticosteroid tapering. If corticosteroid use does not improve, increase the corticosteroid dose to 2 mg/kg/d of (methyl) prednisolone or an equivalent drug and permanently stop ICIs. For patients with grade 3 or 4 elevation of transaminase or total bilirubin, ICIs are permanently discontinued and corticosteroid therapy is administered with an initial dose of 1 to 2 mg/kg/d (methyl) prednisolone or other equivalent drug (21).
Pulmonary toxicities
Approximately 4% of patients developed pneumonitis with anti-PD-1/PD-L1 mAbs, according to a meta-analysis by Nishino et al. Patients who received anti-PD-1 antibodies are more likely to develop immune-related pneumonia at any level (1–5% vs. <1%) than patients who received anti-CTLA-4 antibodies (22). The median time of onset of pneumonitis is considerably later in case of pembrolizumab (19 weeks; range, 0.3–84 weeks) compared to nivolumab (9 weeks; range, 4–26 weeks) (4). Although the incidence of pneumonia is relatively low, the symptoms of patients with pneumonia can rapidly deteriorate, leading to death possibly (23). Therefore, such patients need to be paid close attention. Immune-associated toxic side effects of pneumonia are mainly upper respiratory tract infection, cough (usually manifested as persistent dry cough) or breathing difficulties, lacking of clinical specific characteristics. If such abnormalities are found clinically, routine imaging examinations such as chest X-ray and CT should be performed. Immunotherapeutic pneumonia may present as nonspecific interstitial pneumonia, allergic pneumonia, acute interstitial pneumonia, or cryptogenic pneumonia. If still uncertain, bronchoscopy and bronchoalveolar lavage (BAL) should be performed (24).
For grade 1–2 ICI related pneumonia, treatment includes oral steroid prednisone 1 mg/kg/d or equivalent when infectious pneumonia is excluded. For grade 3 to 4 cases, admission should include a large intravenous dose of corticosteroid [(methyl) prednisolone 2 to 4 mg/kg/d or equivalent] and a permanent discontinuation of immunotherapy. After 2 days, if no improvement is found, immunosuppressive therapy should be added, either infliximab, mycophenolate mofetil (MMF) or cyclophosphamide (5,25).
Endocrine toxicities
Hypothyroidism
In patients with anti-PD-1/PD-L1 mAbs, the incidence of hypothyroidism is 6.6%, according to a recent meta-analysis (26). Thyroid dysfunction usually occurs early in treatment, with a median onset of 6 weeks after the first treatment. In a published study that prospectively monitored thyroid function in melanoma patients treated with pembrolizumab, most patients with hyperthyroidism subsequently developed hypothyroidism within 1–3 months (27). Endocrine events—their median time to onset is varying from 4 to 18 weeks (4). Patients usually have no specific symptoms of discomfort. Clinicians should perform thyroid biochemical tests, including free T4, free T3 and TSH, when hypothyroidism is happened. In severe cases, central hypothyroidism needs to be excluded first, which can take place either independently or as part of hypophysitis (28). Patients with fatigue or other hypothyroid-related complaints should consider thyroid hormone replacement therapy (HRT) (21).
Hyperthyroidism
Endocrine toxicities are common in adverse events reported with ICIs. All endocrine glands could be affected during immunotherapy, but the pituitary glands, thyroid and adrenal are the most common. Barroso-Sousa et al. reported that anti-PD-1 mAbs had the higher prevalence than anti-PD-L1 mAbs, based on the mixed-effects model, the overall incidence of hyperthyroidism is estimated to be 2.9% [95% confidence interval (CI), 2.4–3.7%] (26). Hyperthyroidism is often associated with insomnia, tachycardia, diarrhea, tremors, hyperhidrosis and even exophthalmos. Blood tests show low levels of thyroid stimulating hormone (TSH), normal or high T3 (Triiodothyronine) and/or T4 (thyroxine). Sometimes anti-thyroid peroxidase antibodies and/or thyroid-stimulating immunoglobulin can be found in peripheral blood. Patients with hyperthyroidism need to be treated with β-blockers (propranolol or atenolol) and it is rare to be treated with carbimazole or steroid hormones (21).
Hypophysitis
It is reported that the incidence of hypophysitis is greatest at 6.4% with combination therapy; 3.2% with anti-CTLA-4 agents; 0.4% with anti-PD-1 agents; and <0.1% with anti-PD-L1 agents (26). Patients can present with nausea, headache, vomiting, loss of libido, fatigue, muscle weakness or orthostatic hypotension. Mild hyponatremia is always with low levels of blood adrenocorticotropin (ACTH) and TSH. Other relative hormones, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH) or prolactin could be normal. Sometimes, a magnetic resonance imaging (MRI) of the pituitary gland resection should be performed (29). Once the diagnosis is grade 2 or above, ICIs treatment should be interrupted immediately. Patients with headaches and other neurological symptoms need to be treated with large doses of steroids, and HRT requires long-term maintenance (21).
Adrenal gland alterations
Primary adrenal insufficiency was rare in patients treated with ICIs (30). Primary adrenal insufficiency patients often experienced nausea/vomiting, fatigue, weight loss or skin pigmentation. If necessary, adrenal cortex antibodies and anti-21-hydroxylase should be performed (31). Adrenal crisis is always a medical emergency and intravenous rehydration and corticosteroids should be started immediately (21).
Diabetes
In terms of gene association, CTLA-4 polymorphisms are associated with an increased risk of autoimmune diseases such as type 1 diabetes (T1D). Preclinical models have shown that anti-CTLA-4 can increase the risk of autoimmune diabetes (32). The PD-1 pathway also plays a role in autoimmune diabetes, because in mouse models, blocking the PD-1/PD-L1 axis, mediated by specific CD8 T cells, also leads to T1D (21). Patients with polyphagia, increased urine frequency and volume after immunotherapy should be excluded from diabetes. Diabetes induced by ICIs are the same diagnostic criteria as the primary diabetes (33). Patients with grade 3 to 4 T1D (ketoacidosis) should be immediately admitted to the hospital and begin treatment as newly developed T1D (21).
Cardiotoxicities
Myocarditis
Myocarditis may occur in less than 1% of patients (34). Immune-related toxicity can be manifested as fulminant myocarditis with serious toxic effects and high mortality (approximately 40%) with anti-PD-1/PD-L1 agents (35,36). The symptoms vary from dyspnea, chest pain, palpitations and arrhythmias to pleural effusion or pericardial effusion. Levels of brain natriuretic peptide (BNP) and serum troponin in blood tests could be elevated, and a range of cardiac imaging tests should also be used in patients. Besides, cardiac MRI is better than echocardiogram to reveal cardiac lesions (37). Nevertheless, the gold standard for diagnosis is endomyocardial biopsy.
Specific guidelines for the treatment of immune-mediated myocarditis have not yet been published (1). High doses of corticosteroids have been successfully used to treat cardiac side effects and should be rapidly applied if ICIs-induced cardiac AEs are suspected. If symptoms do not respond quickly to steroids, other immunosuppressive drugs, such as infliximab, MMF, and anti-thymocyte globulin (ATG) may be necessary (21).
Rhythm disturbance
Isolated arrhythmias can occur in patients with structural heart disease receiving anti-PD-1/PD-L1 mAbs (38). When patients develop arrhythmias, immune-mediated toxicity should be excluded first because of their potentially lethality. The physician should place the patients in the intensive care unit (ICU) and perform cardioversion under electrocardiogram. Antiarrhythmic drugs are expectant treatment. Methylprednisolone 1–2 mg/kg may be useful in severe disturbances (5).
Pericarditis
Rare patients may develop autoimmune pericarditis with anti-PD-1/PD-L1 agent. Common symptoms are fever, chest pain, orthopnoea and typical pericardial friction. As far as we know, cardiac tamponade is rare and only seen in rare case reports (39). Colchicine and prednisone may be useful.
Neurological disorders
Demyelinating polyradiculoneuropathy
It has been reported that demyelinating polyradiculopathy resembles Guillain-Barré syndrome in anti-PD-1 treatment. It generally takes 4 weeks from the onset of medication to the onset of symptoms which are usually sensory loss, paresthesia, dysarthria, impaired vision and diplopia. Conduction deceleration can be observed on electromyogram (EMG) nerve. In order to exclude brain metastases, cerebral MRI is also necessary. And the specific signs of albumin-cytologic dissociation can be detected in cerebrospinal fluid (CSF) (40). Since this condition can be life-threatening, aggressive approaches, including intravenous immunoglobulin and/or high doses of methylprednisolone, may be considered in addition to intensive care preparation for ventilator support (5).
Encephalitis
Encephalitis in combination with anti-PD-1/PD-L1 drugs is a rare adverse event which can present as fatigue, confusion, vomiting, fever and convulsive tremor. Brain MRI, CSF analysis and electroencephalography can show some non-specific signs. Cerebellar symptoms could be gait disturbance, altered movements and tremor (41). Prior to obtaining microbiological results, empirical broad-spectrum antibiotics and antiviral therapy can be initiated if immune-related encephalitis is highly suspected, intravenous immunoglobulin may be used in some severe cases (5).
Myasthenia gravis and myasthenia-like syndromes
As for anti-PD-1/PD-L1-induced myasthenia gravis, there is no cranial nerve enhancement or leptomeningeal or parenchymal alterations on brain MRI, however, pathological fibrillation can be observed in single-fibre EMG. It’s necessary to test serum anti-muscle-specific kinase antibodies and anti-acetylcholine receptor and roll out the possible concomitant presence of a thymoma. Owing to myasthenia gravis can lead to respiratory paralysis, clinicians should pay more attention (42). Corticosteroids and/or other inhibitors (azathioprine, cyclosporine or MMF) may be an effective option (5).
Hematotoxicities
Aplastic anemia
It is an uncommon side effect of immunotherapy compared with chemotherapy (43).There are many method to diagnose aplastic anemia, blood tests, flow cytometry and a bone marrow biopsy. Either in nivolumab monotherapy or in combination with ipilimumab, immune treatment-associated aplastic anemia leading to death has been reported (44), doctors must not take it lightly. Transfusion, use of granulocyte colony stimulating factor (G-CSF) or platelet transfusion should be considered in a case-by-case situation under the guidance of the affected cell line. In refractory cases, the use of ATG may be considered (5).
Autoimmune hemolytic anemia (AHIA)
It has been reported that in anti-PD-1 induced AHIA patients, there are usually showing reticulocyte count, lactate dehydrogenase, and reduced haptoglobin in serum, elevated bilirubin, direct Coombs test positive for Immunoglobulin G (IgG) or Complement component 3 (C3) and spherocytosis in the peripheral blood smear (45). We could consider mycophenolate, cyclosporine, cyclophosphamide, azathioprine or intravenous immunoglobulins for severe situations (5).
Immune thrombocytopenic purpura
It has been reported that idiopathic thrombocytopenia purpura (ITP) can occur during anti-PD-1 therapy. It shows that platelet-associated IgG increased, platelet count decreased, normal levels white blood cell and hemoglobin. Besides, megakaryocytes are increased and the proportion of immature platelets is high, bone marrow biopsy presents no abnormal cells (46). Once ITP is suspected, methylprednisolone 1–2 mg/kg should be initiated. Intravenous immunoglobulin, rituximab, or thrombopoietin may be considered in some severe cases (5).
Ocular syndromes
Uveitis
Adverse events associated with ophthalmic immunity are infrequent, affecting only 1% of patients and occurring primarily in patients receiving CTLA-4 inhibitor (47). Uveitis induced by PD-1/PD-L1 inhibitor is characterized by redness of the conjunctiva, blurred vision, photophobia, flossing and sore eyes. If highly suspected, a series of ophthalmologic examination should be considered, for example optical coherence tomography, fundus examination, ultrasonography, fluorescence angiography and electrophysiological examination (48). For mild to severe cases, topical corticosteroids and mydriatic agents may be considered. For severe posterior uveitis cases, transscleral cryotherapy and vitrectomy may be an option (5).
Vogt-Koyanagi-Harada syndrome
It has been reported that patients treated with nivolumab may occur Vogt-Koyanagi-Harada syndrome (uveomeningitis syndrome), a multisystemic disorder. It usually presents with exudative retinal detachment, blurred vision, cutaneous and neurological manifestations. Mydriatic agents can be considered for the ophthalmologic changes (49).
Other ocular toxicity
Uveal effusion has relative with the treatment of anti-PD-1 mAbs. Clinical manifestation is blurry vision, ophthalmodynia and redness after 3–8 weeks’ immunotherapy. The B-scan ultrasonographic image and spectral optical coherence tomography can confirm the diagnosis (50). Once PD-1/PD-L1 inhibitors secondary retinopathy occurs, we can suffer from blurry vision, and clinicians must role out cancer-associated retinopathy (51).
Conclusions
As the arising using of ICIs, tumor treatment has come into an immunotherapy time, especially in NSCLC, and more severe and rare toxicities are being recognized. The frequency of irAEs depends largely on the drugs used, but also on the specific characteristics of the individual patient. Most of the irAEs occur within 2 months of the start of treatment, but they can occur at any time after treatment, so require special attention from doctors. This paper mainly describes the toxicities of different drugs,as for irAEs among different tumors, according to a review on Nature Reviews Clinical Oncology, the frequency mainly depends on the type of drug used (52). An article published on JAMA Oncology also found that there was no association between the tumor type and the incidence of ICIs-induced thyroid dysfunctions (26). However, melanoma patients are more prone to vitiligo after administration of ipilimumab than other tumors (21). The irAEs profile of different immunotherapy drugs is slightly different, which may be due to the different mechanisms of action of different drugs. Treatment methods are briefly discussed in this paper. For more detailed toxicity classification and treatment specifications, please refer to relevant authoritative guidelines. Although most immune-related toxicities are manageable, they may affect the treatment process, even threaten the patients’ life. Therefore, accurate prediction, timely diagnosis and early intervention are conducive to make immunotherapy work better. With the approval of a variety of immunotherapy drugs on the market, we also have new challenges. The timing and sequence of use immunotherapy agents, combination of immunotherapy agents with target therapy and/or chemotherapy and the survival outcomes still need to be further clarified.
Acknowledgments
Funding: This study was supported in part by a grant from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National Key Research & Development Project (2016YFC0902300). Major disease clinical skills enhancement program of 3-year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), grant of Shanghai Science and Technology Commission (16JC1405900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Baraibar I, Melero I, Ponz-Sarvise M, et al. Safety and Tolerability of Immune Checkpoint Inhibitors (PD-1 and PD-L1) in Cancer. Drug Saf 2019;42:281-94. [Crossref] [PubMed]
- Khandekar MJ, Jain R. Smooth sailing for immunotherapy for unresectable stage III non-small cell lung cancer: the PACIFIC study. Transl Cancer Res 2018;7:S16-20. [Crossref] [PubMed]
- Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [Crossref] [PubMed]
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7-18. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Zoratti MJ, Devji T, Levine O, et al. Network meta-analysis of therapies for previously untreated advanced BRAF-mutated melanoma. Cancer Treat Rev 2019;74:43-8. [Crossref] [PubMed]
- Weber J. Overcoming Immunologic Tolerance to Melanoma: Targeting CTLA-4 with Ipilimumab (MDX-010). Oncologist 2008;13:16-25. [Crossref] [PubMed]
- Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71:161-9. [Crossref] [PubMed]
- Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018;67:2056-67. [Crossref] [PubMed]
- Read S, Greenwald R, Izcue A, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006;177:4376-83. [Crossref] [PubMed]
- Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [Crossref] [PubMed]
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190-209. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Gonzalez RS, Salaria SN, Bohannon CD, et al. PD-1 inhibitor gastroenterocolitis: case series and appraisal of 'immunomodulatory gastroenterocolitis'. Histopathology 2017;70:558-67. [Crossref] [PubMed]
- Cramer P, Bresalier RS. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr Gastroenterol Rep 2017;19:3. [Crossref] [PubMed]
- Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017;66:581-92. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Mekki A, Dercle L, Lichtenstein P, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer 2018;96:91-104. [Crossref] [PubMed]
- Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists' perspective. J Clin Pathol 2018;71:665-71. [Crossref] [PubMed]
- Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-iv42. [Crossref] [PubMed]
- Chuzi S, Tavora F, Cruz M, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017;9:207-13. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Castanon E. Anti-PD1-Induced Pneumonitis: Capturing the Hidden Enemy. Clin Cancer Res 2016;22:5956-8. [Crossref] [PubMed]
- Possick JD. Pulmonary Toxicities from Checkpoint Immunotherapy for Malignancy. Clin Chest Med 2017;38:223-32. [Crossref] [PubMed]
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:173-82. [Crossref] [PubMed]
- de Filette J, Jansen Y, Schreuer M, et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab 2016;101:4431-9. [Crossref] [PubMed]
- Gonzalez-Rodriguez E, Rodriguez-Abreu D. Spanish Grp Canc I-B. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016;21:804-16. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714. [Crossref] [PubMed]
- Ariyasu R, Horiike A, Yoshizawa T, et al. Adrenal Insufficiency Related to Anti-Programmed Death-1 Therapy. Anticancer Res 2017;37:4229-32. [PubMed]
- Paepegaey AC, Lheure C, Ratour C, et al. Polyendocrinopathy Resulting From Pembrolizumab in a Patient With a Malignant Melanoma. J Endocr Soc 2017;1:646-9. [Crossref] [PubMed]
- Lühder F, Höglund P, Allison JP, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med 1998;187:427-32. [Crossref] [PubMed]
- Amer Diabet A. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S13-27. [Crossref] [PubMed]
- Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 2018;19:E447-58. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579-89. [Crossref] [PubMed]
- Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017;136:2085-7. [Crossref] [PubMed]
- Behling J, Kaes J, Muenzel T, et al. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res 2017;27:155-8. [Crossref] [PubMed]
- Asai M, Kato Y, Kawai S, et al. Management of cardiac tamponade during nivolumab of lung cancer with intrapericardial bleomycin: case report. Immunotherapy 2019;11:467-72. [Crossref] [PubMed]
- Nukui T, Nakayama Y, Yamamoto M, et al. Nivolumab-induced acute demyelinating polyradiculoneuropathy mimicking Guillain-Barre syndrome. J Neurol Sci 2018;390:115-6. [Crossref] [PubMed]
- Levine JJ, Somer RA, Hosoya H, et al. Atezolizumab-induced Encephalitis in Metastatic Bladder Cancer: A Case Report and Review of the Literature. Clin Genitourin Cancer 2017;15:e847-9. [Crossref] [PubMed]
- Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol 2018;137:601-9. [Crossref] [PubMed]
- Michot JM, Vargaftig J, Leduc C, et al. Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer 2017;80:1-4. [Crossref] [PubMed]
- Comito RR, Badu LA, Forcello N. Nivolumab-induced aplastic anemia: A case report and literature review. J Oncol Pharm Pract 2019;25:221-5. [Crossref] [PubMed]
- Shaikh H, Daboul N, Albrethsen M, et al. A case of autoimmune haemolytic anaemia after 39 cycles of nivolumab. BMJ Case Rep 2018. [Crossref] [PubMed]
- Jotatsu T, Oda K, Yamaguchi Y, et al. Immune-mediated thrombocytopenia and hypothyroidism in a lung cancer patient treated with nivolumab. Immunotherapy 2018;10:85-91. [Crossref] [PubMed]
- Dalvin LA, Shields CL, Orloff M, et al. CHECKPOINT INHIBITOR IMMUNE THERAPY: Systemic Indications and Ophthalmic Side Effects. Retina 2018;38:1063-78. [Crossref] [PubMed]
- Kanno H, Ishida K, Yamada W, et al. Uveitis induced by programmed cell death protein 1 inhibitor therapy with nivolumab in metastatic melanoma patient. J Infect Chemother 2017;23:774-7. [Crossref] [PubMed]
- Arai T, Harada K, Usui Y, et al. Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol 2017;44:975-6. [Crossref] [PubMed]
- Thomas M, Armenti ST, Ayres MB, et al. Uveal Effusion After Immune Checkpoint Inhibitor Therapy. JAMA Ophthalmol 2018;136:553-6. [Crossref] [PubMed]
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [Crossref] [PubMed]
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563-80. [Crossref] [PubMed]


