Rationale and design of a phase II trial of osimertinib as first-line treatment for elderly patients with epidermal growth factor receptor mutation-positive advanced non-small cell lung cancer (SPIRAL-0 study)
Introduction
Due to population ageing, the incidence of lung cancer is increasing. The highest incidence rates of lung cancer in the US have been observed in the 80–89-year age group in men and 80–84-year age group in women (1). Moreover, 14% of lung cancer patients in the US are aged ≥80 years (2). In Japan, 45,000 individuals aged ≥75 years were estimated to have died of lung cancer in 2016 (3). Thus, the development of a treatment strategy for elderly patients with NSCLC is an important issue.
Gefitinib, erlotinib are first-generation, and afatinib is a second-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), which have been approved for the treatment of EGFR mutation-positive advanced NSCLC. A meta-analysis of randomized control trials (RCTs) showed that EGFR-TKIs demonstrated a significantly longer median progression-free survival (PFS) than chemotherapy (11 versus 5.6 months) (4). The third-generation EGFR-TKI, osimertinib, has been shown to have a potent inhibitory effect against both sensitizing mutations and resistance by T790M mutations in EGFR, with a relatively low affinity for wild-type EGFR. Osimertinib has been approved for patients with T790M mutation. As a first-line treatment, osimertinib significantly prolonged PFS in comparison with the standard EGFR-TKIs in the FLAURA study [18.9 versus 10.2 months; hazard ratio (HR): 0.46] (5).
A phase II trial demonstrated that the efficacy of gefitinib is similar in younger (<75 years) and elderly (≥75 years) patients with EGFR-positive patients (6). Osimertinib is well-tolerated compared with first-generation EGFR-TKIs (7). The major adverse events observed in the FLAURA study were diarrhea (58% at all Grades, 2% at ≥ Grade 3) and acneiform rash (58% at all Grades, 1% at ≥ Grade 3) in the osimertinib group, and, in a similar manner, diarrhea (57% at all Grades, 2% at ≥ Grade 3) and acneiform rash (78% at all Grades, 7% at ≥ Grade 3) in the first-generation EGFR-TKIs group. The incidence of adverse events of ≥ Grade 3 was reported to be lower in the osimertinib group (33.7%) compared with the first-generation EGFR-TKIs group (44.8%) (5).
Based on these findings, osimertinib is considered to potentially provide benefits to EGFR mutation-positive patients. In Japan, lung cancer clinical practice guidelines define patients aged ≥75 years as ‘elderly’; furthermore, based on the results of some clinical studies in Japan, gefitinib and erlotinib are recommended in patients aged ≥75 years (6,8). Although a subset analysis of the data of patients aged ≥65 years was performed in the FLAURA trial, the data obtained for patients aged ≥75 years is highlighted in the elderly patients. In this study, we aim to investigate the efficacy and safety of osimertinib as a first-line treatment for EGFR mutation-positive NSCLC in patients aged ≥75 years.
Methods
Study design
This is a single arm, prospective, open-label, multicenter, phase II trial. The present study begun in November 2018 and is currently in progress.
End points
The primary endpoint is one-year progression-free survival (PFS). Secondary endpoints are response rate (RR), progression-free survival (PFS), overall survival (OS), and safety.
Eligibility criteria
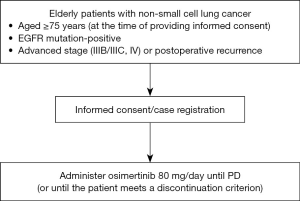
The inclusion and exclusion criteria are shown in Table 1.

Full table
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; AST, aspartate amino transferase; ALT, alanine aminotransferase; SpO2, peripheral capillary oxygen saturation.
Dose and treatment regimen
Osimertinib will be administered at a dose of 80 mg orally once daily, at the same time, with or without food. If a scheduled dose is missed, it will be recommended to take osimertinib as soon as possible; however, if it is less than 12 hours until the next scheduled dose, the missed dose of osimertinib will be omitted. Patients will continue to receive oral osimertinib until disease progression or until a discontinuation criterion (Table 2) is met. A schema of the study is shown in Figure 1.

Full table
Rationale for the setting of the number of enrolled subjects
In the FLAURA study, 1-year PFS for EGFR mutation-positive patients treated with osimertinib was approximately 70% and the median PFS was 18.9 months (5). In addition, the 1-year PFS for EGFR mutation-positive elderly patients who received gefitinib in Japan was reported to be approximately 50% and the median PFS to be 12.1 months (6). Therefore, 37 patients are required for the present study, when calculated based on normal approximation with a one-sided α level of 5% and 80% power, assuming that the expected 1-year PFS is 70% and the 1-year PFS threshold is 50%. Thus, to account for possible dropouts, the study sample size has been set to 40.
Population to be analyzed
Patients enrolled in this study, excluding those with serious protocol violation, will be included in the full analysis set (FAS). The per-protocol set (PPS) will include the remaining subjects from the FAS, after excluding those with violation of inclusion/exclusion criteria or violation for prohibited concomitant drugs/therapies. The safety analysis set (SAF) will include patients who received ≥1 dose of osimertinib.
Statistical analysis
The 1-year PFS will be estimated by the Kaplan-Meier method, and the confidence interval (CI) calculated using the Greenwood method. If the lower limit of the estimated 90% CI exceeds the threshold of 50%, it will be considered statistically significant. As for PFS and OS, the Kaplan-Meier method will be used to estimate the survival curve, median, and annual rate. The Brookmeyer and Crowley method will be used to estimate the median CI, and Greenwood’s method will be used to estimate the standard error of the annual rate. The RR and 95% CI (two-sided) will be estimated via the Wilson method. The severity and incidence of each adverse event will be recorded.
Discussion
Osimertinib is expected to provide well-tolerated treatment for elderly EGFR mutation-positive patients with fewer serious adverse drug reactions and longer survival benefit compared with first and second generation EGFR-TKIs. To our knowledge, this study is the first prospective trial to investigate the efficacy and safety of osimertinib as a first-line treatment for EGFR mutation-positive lung cancer in patients aged ≥75 years.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Funding: This work was supported by “Externally sponsored scientific research” in AstraZeneca (ESR-17-13222).
Footnote
Conflicts of Interest: J Uchino reports grants from Eli Lilly Japan K.K. that are outside of the submitted work. T Yamada reports grants from Nippon Boehringer Ingelheim and Ono Pharmaceutical Company that are outside of the submitted work. K Takayama reports grants from Chugai-Roche and Ono Pharmaceutical Company, personal fees from AstraZeneca K.K., Chugai-Roche, MSD-Merck, Eli Lilly, Boehringer-Ingelheim, and Daiichi-Sankyo that are outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol and informed consent documents were approved by the ethical committees of the participating institutions, Clinical Research Network Fukuoka Certified Review Board (No. 18-C06), and informed consent was obtained from all patients.
References
- Bravo-Iñiguez C, Perez Martinez M, Armstrong KW, et al. Surgical resection of lung cancer in the elderly. Thorac Surg Clin 2014;24:371-81. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Center for Cancer Control and Information Services, National Cancer Center, Japan Web site. Available online: Accessed November 1, 2018http://ganjoho.jp/professional/statistics/statistics.html/
- Lee CK, Davies L, Wu YL, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: Individual patient data meta-analysis of overall survival. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 2012;7:1417-22. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. OSIMERTINIB, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Goto K, Nishio M, Yamamoto N, et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 2013;82:109-14. [Crossref] [PubMed]