
Extent of resection and lymph node evaluation in early stage metachronous second primary lung cancer: a population-based study
Introduction
Lung cancer is one of the most prevalent and deadliest cancers in the world, and non-small cell lung cancer (NSCLC) is the commonest form of lung cancer (1). Fortunately, since low-dose computed tomography has proven to be a better screening method, more and more cases of lung cancer have been detected in early stage and curatively resected (2). According to the prognostic data of the 8th edition of the American Joint Committee on Cancer (AJCC) TNM stage, the 5-year survival rate of the earliest stage NSCLC has reached as high as 90% (3). However, these cured survivors constitute a population at high risk to develop a second primary lung cancer (SPLC), and several studies have highlighted the importance of continuous surveillance in these patients (4-6).
For early stage metachronous SPLC with adequate pulmonary function reserve, surgery is the preferred treatment according to the National Comprehensive Cancer Network (NCCN) and the American College of Chest Physicians (ACCP) guidelines (7,8). However, the extent of resection remains highly controversial. Several retrospective studies have compared lobectomy with sublobar resection in these patients, but demonstrated conflicting results. Some have believed that sublobar resection provides comparable long-term survival with improved perioperative morbidity (9,10). However, others have argued that lobectomy, as an anatomic resection, is associated with better disease control and therefore longer survival (11,12).
Lymph node evaluation is an indispensable component in lung cancer resection and complete resection requires systematic lymph node sampling or dissection (7). Previous studies have demonstrated that the number of examined lymph node is an important aspect of thorough lymph node evaluation and may be closely related to survival (13,14). However, data on lymph node evaluation during SPLC surgery has been scarce and currently no guideline or consensus has addressed this important topic.
In this study, we utilized the Surveillance, Epidemiology, and End Results (SEER) database to identify early stage metachronous SPLC patients, and we aimed to compare the survival outcomes of different extents of resection and lymph node evaluation in these patients.
Methods
Study population
The study population was selected from 18 SEER Registries (November 2018 submission, 2000–2016) with multiple primary standardized incidence ratios (MP-SIR) session. According to the slightly modified Martini & Melamed diagnosis criteria for SPLC proposed by the ACCP guideline (8,15,16), SPLC was diagnosed when any of the following conditions was met: (I) different histology or arising from separate foci of carcinoma in situ; (II) same histology, tumor in different lobe as primary without any N2/N3 involvement or systemic metastases; (III) same histology with at least 4 years interval between initial primary lung cancer (IPLC) and SPLC without systemic metastases. Cases of small cell carcinoma, unknown cause of death, unknown lesion location, SPLC received local treatment except surgery, pneumonectomy, or unknown surgery were excluded. In this study, we focused on early stage metachronous SPLC patients who had received lobectomy for IPLC; thus, patients with an interval between IPLC & SPLC of more than 3 months were selected while patients with nodal or distant metastasis were excluded.
Patients characteristics and end points
Information regarding patients’ baseline demographics, tumor characteristics, treatment, and survival was collected from SEER. International Classification of Diseases for Oncology (3rd edition) morphology codes were extracted and tumor histology was classified according to the 2015 World Health Organization Classification of Lung Tumors (17). Extents of resection were categorized as sublobar resection and lobectomy. Sublobar resection included wedge resection, segmentectomy, and other resection of less than one lobe. Lobectomy was defined as resection of one or two lobes but less than the whole lung. The interval between IPLC and SPLC, and extent of lymph node evaluation were dichotomized based on cutoff value from previous studies (8,14). Meanwhile, age and tumor size were dichotomized by their respective medians.
The primary outcome was overall survival (OS) and the secondary outcome was lung cancer-specific survival (CSS). Survival months were calculated from the time of SPLC diagnosis to the time of death or the last follow-up. All patients were followed up to December 31st, 2016; patients who were alive on the last follow-up were censored. Additionally, causes of death other than lung cancer were censored in the CSS analysis.
Statistical analysis
Pearson chi-square test or Fisher’s exact test was used to compare the difference between groups. Multiple comparisons were adjusted by Bonferroni correction. The Kaplan-Meier method was applied in survival analysis and survival curves were compared by log-rank test. Potential statistically significant factors (P<0.10) from univariate survival analysis were identified and selected into the Cox proportional hazards regression model for multivariate survival analysis. The Cox regression model was developed by forward stepwise selection (likelihood-ratio) with entry/removal probability as 0.05/0.10 respectively. A two-sided P value <0.05 was considered statistically significant.
All statistical analysis was conducted by IBM SPSS statistics version 25, and the survival curves were drawn by R version 3.6.1.
Results
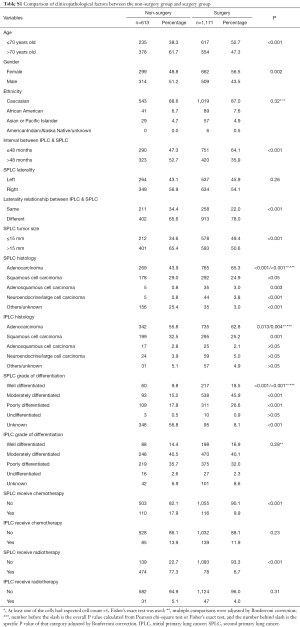
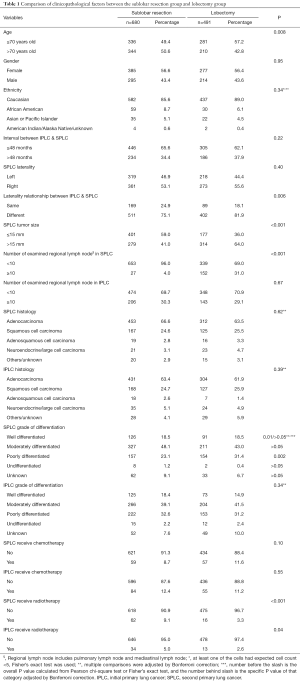
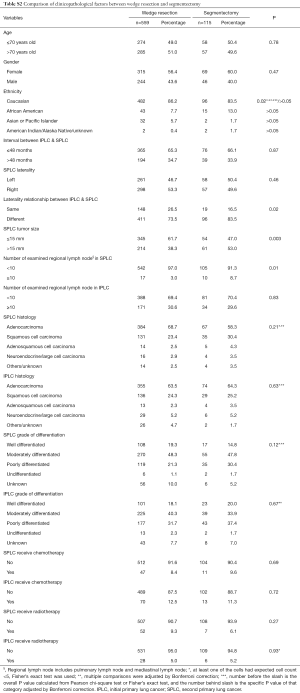
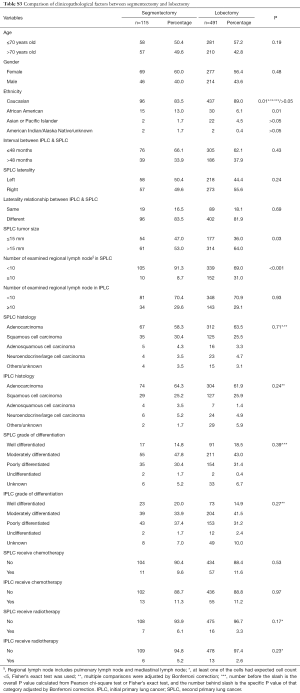
The selection flow is presented in Figure S1. A total of 1,784 early stage metachronous SPLC patients, including 613 without surgery and 1,171 with surgery, were identified. The median follow-up time, OS, and CSS were 41, 56, and 84 months respectively, and the median interval between IPLC and SPLC was 40 months. Relevant clinicopathological factors were compared between the surgery group and non-surgery group. Notably, patients in surgery group were more likely to be younger, to have a shorter interval between IPLC and SPLC, SPLC contralateral to IPLC, and SPLC of smaller size (Table S1). Compared with sublobar resection, lobectomy group patients were more likely to be younger, to have SPLC contralateral to IPLC, SPLC of a larger size and more lymph nodes examined (Table 1). Within the sublobar resection group, 559 patients received wedge resection, 115 patients received segmentectomy, and 6 patients received other resection of less than one lobe. Compared with wedge resection, surgeons were more inclined to perform segmentectomy in SPLC contralateral to IPLC and SPLC with a larger tumor size. Moreover, segmentectomy was associated with significantly more lymph nodes examined than wedge resection (median of examined lymph node number: segmentectomy 2, sublobar resection 0, P=0.01, Table S2). Furthermore, compared with lobectomy, surgeons were more likely to perform segmentectomy in African Americans and SPLC of a smaller size. In addition, segmentectomy was associated with significantly less lymph nodes examined than lobectomy (median of examined lymph node number: segmentectomy 2, lobectomy 5, P<0.001, Table S3).

Full table
Full table
Full table
Full table
Both sublobar resection and lobectomy groups had a significantly longer OS and CSS compared with the non-surgery group (Figure 1A,B, all pairwise P<0.001). In addition, compared with sublobar resection, the lobectomy group had longer OS (HR: 0.83, 95% CI: 0.71–0.97, P=0.02, Figure 1A) and CSS (HR: 0.72, 95% CI: 0.60–0.88, P=0.001, Figure 1B). Furthermore, lobectomy demonstrated consistent OS (HR: 0.75, 95% CI: 0.57–0.97, P=0.03, Figure S2A) and CSS (HR: 0.64, 95% CI: 0.47–0.87, P=0.01, Figure S2B) benefit even when compared with segmentectomy. When limited within sublobar resection, there was no statistically significant difference between wedge resection and segmentectomy in both OS (P=0.29, Figure S3A) and CSS (P=0.28, Figure S3B).

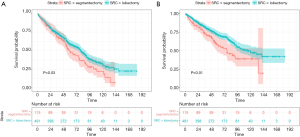

The effect of examined lymph node number on survival was also investigated in the surgery group. Examined lymph node number ≥10 consistently demonstrated superior OS (HR: 0.63, 95% CI: 0.50–0.81, P<0.001, Figure 2A) and CSS (HR: 0.54, 95% CI: 0.40–0.74, P<0.001, Figure 2B) when compared with examined lymph node number <10.

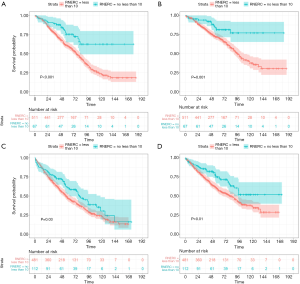
In the subgroup analysis, the surgery group was further divided into tumor size of SPLC ≤15 and >15 mm. When tumor sizes were ≤15 mm, even if there was no statistically significant difference in OS (median OS: lobectomy 87 months; sublobar resection: 77 months; P=0.12, Figure 3A), lobectomy was associated with better CSS compared with sublobar resection (HR: 0.63, 95% CI: 0.45–0.87, P=0.01, Figure 3B). When tumor sizes were >15 mm, lobectomy demonstrated consistently superior OS (HR: 0.73, 95% CI: 0.59–0.90, P=0.003, Figure 3C) and CSS (HR: 0.67, 95% CI: 0.53–0.86, P=0.002, Figure 3D). As for regional lymph node examination (Figure 4), examined lymph node number ≥10 consistently demonstrated longer OS (≤15 mm, HR: 0.42, 95% CI: 0.26–0.68, P<0.001; >15 mm, HR: 0.72, 95% CI: 0.54–0.96, P=0.03, Figure 4A,C) and CSS (≤15 mm, HR: 0.37, 95% CI: 0.20–0.68, P=0.001; >15 mm, HR: 0.60, 95% CI: 0.42–0.87, P=0.01, Figure 4B,D) regardless of tumor size.

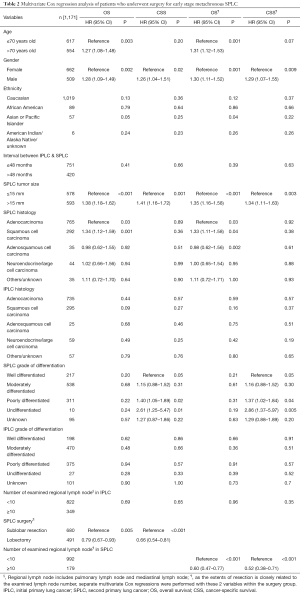
In univariate survival analysis, older age, male gender, SPLC of a larger size, SPLC without surgery and SPLC with less examined lymph node number were high risk factors for poorer survival in early stage metachronous SPLC. On the other hand, SPLC of adenocarcinoma and well differentiated grade were associated with better survival (Table S4). As the extent of resection is closely related to the examined lymph node number, separate multivariate Cox regressions were performed with these 2 variables within the surgery group. Male gender and SPLC of a larger size were associated with poorer survival. And notably, patients with lobectomy and more lymph nodes examined during SPLC surgery had significantly better survival in multivariate Cox regression (Table 2).
Full table
Full table
Discussion
Current evidence regarding the extents of resection in early stage metachronous SPLC has been limited and controversial. Several retrospective studies showed that sublobar resection provided equivalent survival compared to lobectomy in metachronous SPLC (9,10) while others reported that lobectomy was associated with better survival (11,12). Notably, the level of evidence of these studies was limited by their relatively small sample size. Moreover, these studies included patients who had received pneumonectomy in IPLC, which would greatly limit the cardiopulmonary functional reserve for secondary resection. In this study, we utilized the SEER database, which covers approximately 34% of the US population, to focus on early stage metachronous SPLC, and all selected patients had received standard lobectomy for IPLC. Our study not only confirmed surgery as the preferred treatment for early stage metachronous SPLC, but also demonstrated that lobectomy was associated with significantly better survival compared with sublobar resection.
Previous studies in SPLC (11) and NSCLC (18,19) have demonstrated that segmentectomy, as an anatomical resection, may provide similar outcome to lobectomy and superior outcome to wedge resection. However, in our study, when compared with segmentectomy, lobectomy exhibited superior survival. Moreover, to our surprise, the benefit of lobectomy compared with sublobar resection even extended into smaller tumor size (≤15 mm) lesion, leading to better CSS. No other study has specifically compared different extents of resection in SPLC with small tumor size to our best knowledge, and it is reasonable to assume that a lesser extent of resection may be adequate for a smaller tumor. However, when referring to studies in NSCLC (mostly IPLC), the evidence supporting the superiority of lobectomy in early stage NSCLC with small tumor size has been convincing. A landmark randomized controlled trial by Lung Cancer Study Group demonstrated that sublobar resection increased locoregional recurrence without conferring improved postoperative morbidity and mortality, thus establishing lobectomy as the standard of care for T1N0 NSCLC (20). A SEER study, which included 15,760 T1aN0M0 NSCLC patients, found that even in tumor sizes ≤10 mm, lobectomy provided better survival than sublobar resection (21). Additionally, a National Cancer Database study with 13,606 T1aN0M0 NSCLC patients demonstrated that sublobar resection, including segmentectomy, was associated with positive resection margin, less than 3 lymph nodes examined, and significantly worse survival (22). We believe similar mechanism may also exist in early stage metachronous SPLC, and the superiority of lobectomy mainly derives from a safer resection margin and more lymph nodes examined, which avoids understaging. However, future randomized controlled trials are required to validate the benefit of lobectomy compared with sublobar resection. In addition, sublobar resection also confers survival benefit compared with non-surgery as demonstrated in our study, and remains a feasible alternative in patients with compromised pulmonary function.
Complete resection requires systematic lymph node sampling or dissection (7). Previous studies have demonstrated that examined lymph node number is an important aspect of thorough lymph node evaluation and may be closely related to survival in NSCLC (13,14). Nevertheless, data on lymph node evaluation during SPLC surgery has been scarce, and to our best knowledge, no guideline or consensus has addressed this important issue. Our study indicated that examined lymph node number ≥10 was consistently associated with significantly better survival regardless of tumor size. These findings extend the application of thorough lymph node evaluation to SPLC, and the examination of no less than 10 lymph nodes is recommended during SPLC surgery.
In fact, examined lymph node number is closely associated with the extents of resection as demonstrated in our study. Generally, thorough intralobar and hilar lymph node evaluation are technically difficult for sublobar resection. However, it is possible to combine sublobar resection with thorough lymph node evaluation if the radiological or surgical lymph node evaluation technique is improved. These techniques will undoubted improve the survival of early stage metachronous SPLC patients with limited pulmonary function. Future efforts should therefore focus on a less invasive but more thorough lymph node evaluation technique. Until this becomes available, surgeons should perform lymph node evaluation based on the comprehensive judgment of patients’ status, accompanying surgical risk, and their own experience.
Several limitations exist in this study. First, pulmonary function is not available in the SEER database, and thus we could not determine whether patients with poorer pulmonary function were more likely to receive sublobar resection. In addition, potential pulmonary function preservation related to smaller extent of resection could not be evaluated. Second, the lack of postoperative morbidity and mortality data prevented us from evaluating the safety of different extents of resection and lymph node evaluation. Third, although utilizing a population database, this study is subject to potential bias due to its retrospective nature. Prospective randomized controlled trials are required to ultimately determine the optimal extent of resection and lymph node evaluation.
Conclusions
In conclusion, this population-based study compares the survival outcomes of different extents of resection and lymph node evaluation in early stage metachronous SPLC patients who had received lobectomy for IPLC. And our results indicate that both lobectomy and examined lymph node number ≥10 are associated with significantly better survival. Therefore, lobectomy and thorough lymph node evaluation should be considered for early stage SPLC whenever possible. However, randomized controlled trials are still needed to confirm their effect and safety.
Acknowledgments
Funding: This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors sincerely thank all the staff of the SEER program for their important work and diligent effort.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The approval by Sun Yat-sen University Cancer Center institutional review board and informed consent has been waivered because this study is based on a publicly available database.
Data Sharing Statement: No additional data available.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Lancia A, Merizzoli E, Filippi AR. The 8th UICC/AJCC TNM edition for non-small cell lung cancer staging: getting off to a flying start? Ann Transl Med 2019;7:S205. [Crossref] [PubMed]
- Han SS, Rivera GA, Tammemägi MC, et al. Risk stratification for second primary lung cancer. J Clin Oncol 2017;35:2893-9. [Crossref] [PubMed]
- Wozniak AJ, Schwartz AG. The risk of second primary lung cancer: an unsolved dilemma. Transl Lung Cancer Res 2018;7:S54-6. [Crossref] [PubMed]
- Hanna WC, Paul NS, Darling GE, et al. Minimal-dose computed tomography is superior to chest x-ray for the follow-up and treatment of patients with resected lung cancer. J Thorac Cardiovasc Surg 2014;147:30-3. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-small cell lung cancer (version 2.2019).
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Bae MK, Byun CS, Lee CY, et al. The role of surgical treatment in second primary lung cancer. Ann Thorac Surg 2011;92:256-62. [Crossref] [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;145:683-90; discussion 690-1. [Crossref] [PubMed]
- Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg 2013;44:e120-5; discussion e125.
- Doddoli C, Thomas P, Ghez O, et al. Surgical management of metachronous bronchial carcinoma. Eur J Cardiothorac Surg 2001;19:899-903. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [Crossref] [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [Crossref] [PubMed]
- Detterbeck FC, Jones DR, Kernstine KH, et al. Lung cancer. Special treatment issues. Chest 2003;123:244S-58S. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of segmentectomy and lobectomy in stage IA adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of surgical procedure for patients with non-small-cell lung cancer ≤1 cm or >1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]


