The prognostic effect of TTF-1 expression in the Chinese population of patients with advanced lung adenocarcinomas
Introduction
Lung cancer, for which non-small cell lung cancer (NSCLC) accounts for the largest portion, remains the leading cause of cancer-related mortality worldwide. In the advanced stage of lung adenocarcinoma, the effect of palliative chemotherapy is small. Patients with specific genetic modifications, such as EGFR, ALK, HER2, MET, BRAF, RET and ROS1, however, benefit from new systemic therapies targeted at genetic alteration with better overall survival compared to chemotherapy (1-5). These studies demonstrate that the recent advances in the treatment strategy of advanced NSCLC can significantly improve the poor survival in stage 4 adenocarcinoma of the lung through novel driver oncogene–based precision therapy.
Thyroid transcription factor 1 (TTF-1), with its frequent expression in pulmonary adenocarcinoma (72.1%) (6), is one of the most commonly used immunohistochemical indicators to differentiate adenocarcinoma from squamous cell carcinoma and extra-thoracic adenocarcinomas (7). It is also a special lineage-survival oncogene in lung cancer in line with recent researches (8-10). It has become apparent that TTF-1 expression acts as an antagonist in its biological and clinical functions for tumor progression. Therefore, TTF-1 may represent a relevant predictive and/or prognostic indicator for patients with lung adenocarcinoma though its role in tumour biology.
Lung adenocarcinomas as the subgroup of lung cancers have better responsiveness to pemetrexed (11), especially those harboring RET and ROS1 re-arrangements (12,13). Recent research has found that expression of TTF-1 in the non-Asian population is associated with increased survival in patients with advanced lung adenocarcinomas (14). Nevertheless, in Chinese patients with advanced lung adenocarcinomas, the prognostic benefit of TTF-1 remains unknown. The objective of this study was to examine the relationship between expression and survival of TTF1 in Chinese patients with advanced stage lung adenocarcinoma. Furthermore, we hypothesised that TTF-1 might predict sensitivity pemetrexed-based chemotherapy.
Methods
Patients and regimen
We obtained clinical data from 346 newly diagnosed patients with phase IV adenocarcinomas who received treatment at Qingdao University’s Affiliated Hospital between January 2013 and December 2014. Among them, 102 patients did not have immunohistochemical detection of TTF-1, and 35 patients did not have a continuous systemic treatment in our hospital. Finally, 209 patients with advanced lung adenocarcinoma met our inclusion criteria. We collected demographic and clinicopathologic data from those patients including age at diagnosis, sex, location of carcinoma, date of diagnosis, TTF-1 expression, gene mutation state, grade of differentiation, Karnofsky performance status, smoking history and treatment regimens. Medical records collected clinicopathological data and follow-up information. Overall survival has been estimated from the date of lung adenocarcinoma diagnosis to the date of death, or the date of the last follow-up. We performed the follow-up by phone or by querying the medical records to get the patient’s last point of contact or date of death. The predictive effect of TTF-1 on the response to pemetrexed-based chemotherapy was evaluated in patients receiving pemetrexed-based dual or triple chemotherapy.
TTF-1 expression testing
TTF-1 (D2E8) rabbit mAb#12373 was investigated through immunohistochemistry. For each set, 1 tumor slide was analyzed by two investigators who examined the cases at the same time without having knowledge medically using the multi head viewing microscope and reported as a binary variable (positive was equal to there being a nuclear reaction in the tumor sample tested; negative meant that there was no reaction).
Statistical analysis
We used chi-squared and t-tests to assess the relationship between the expression of TTF-1 and the clinicopathologic characteristics. The effect of TTF-1 expression on specific chemotherapy responses was estimated in patients receiving either the initial, double, or triple chemotherapy based on cisplatin. We evaluated the duration of the treatment to assess effects of the chemotherapy. The Kaplan-Meier approach was used to measure the univariate impact of clinicopathological features and TTF-1 expressions on the overall survival of the patient and to determine the discrepancies in the survival of subgroups compared to the log-rank test of Mantel. A multivariate method was used to study the effects of TTF-1 expression on overall survival using a Cox proportional hazard regression. Because of their associations with TTF-1 expression, the model was modified for the level of distinction, gene mutation status, Karnofsky performance status, smoking history, diagnostic age and gender as confounding factors. Nomogram model was designed to calculate our marker's prognostic value. The concordance index (C-index) measured the efficacy of discrimination of a nomogram model, ranging from 0.5 (no discrimination) to 1 (highest discrimination).A comparison between expected survival outcome and observed survival was tested and the bootstrap technique of 800 repetitions was used for internal verification to assess this model’s validity. SPSS 22.0 software (IBM SPSS Inc. USA) and R version 3.5.1 (R Foundation for Statistical Computing) with ggplot2 and rms statistical packages were analyzed. All P values are based on two-sided statistical analysis and considered significant a P value of <0.05. The Affiliated Hospital of Qingdao University (No. QYFY WZLL 25595) approved this retrospective biomarker study.
Results
Clinical features and the association with TTF-1 expression
We analyzed 209 patients who met our criteria, of which 166 (79%) patients were TTF-1 positive, and 43 (21%) patients were TTF-1 negative. There was no significant difference in the clinical features of the patients with TTF-1 positive tumors and those with TTF-1 negative tumors (Table 1). Sixty patients (28.7%) had positive activation of the EGFR mutation tumors, and among TTF-1 positive and negative patients, the proportion of EGFR mutations was not statistically significant (31.9% vs. 13.2%, P=0.43).
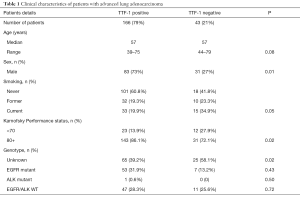
Full table
The univariate relationship between TTF-1 and overall survival in patients
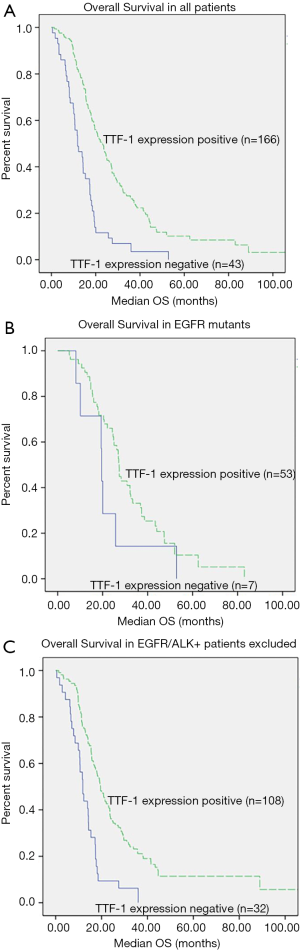
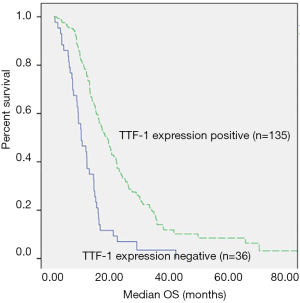
Median survival was significantly prolonged in patients with TTF-1 positive tumors compared to the patients with TTF-1 negative tumors [22.7 vs. 11.8 months, (P<0.0001)] (Figure 1A). Among those with EGFR mutations (n=60), no difference in survival between TTF-1 expression groups and non-TTF-1 expression groups (27.3 vs. 19.5 months, P=0.16, Figure 1B). When excluding patients with negative EGFR or ALK-targeted somatic cell changes (or patients with unbeknown EGFR/ALK status), survival was longer in patients with TTF-1 positive [n=140, 19.5 vs. 11.8 months, (P<0.0001)] (Figure 1C). Among those who were treated with initial doublet or triplet chemotherapy based on cisplatin, both TTF-1 positive and negative were closely related to survival (n=21.3 vs. 11.8 months, P<0.0001, Figure 2).
TTF-1 prognostic effect: the multivariate association with overall survival
We used variables that may be associated with survival, such as age, sex, smoking history, physical status, molecular subsets and treatment received, for multiple regression analysis. After adjusting for these variables, the effect of TTF-1 positive on overall survival (HR 0.42, 95% CI: 0.292–0.606, P<0.0001) exceeded Karnofsky Performance Status ≥80% (HR 0.52, 95% CI: 0.343–0.775, P=0.001) and received combined chemotherapy (HR relative to single-agent chemotherapy 0.742, 95% CI: 0.411–1.339, P=0.321) or targeted therapy (HR relative to single-agent chemotherapy 2.226, 95% CI: 1.07–4.634, P=0.032) (Table 2).
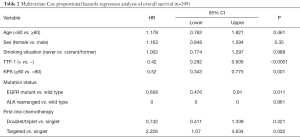
Full table
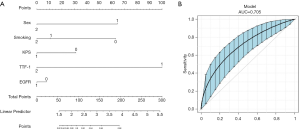
A nomogram based on TTF-1 markers to predict lung cancer survival
The above evidence indicates that TTF-1 was a prognostic factor for lung adenocarcinoma. A nomogram model was built by the combination of TTF-1 and other clinical factors (Figure 3) to assess its function in lung adenocarcinoma. During the variable selection process, the number of variates was restrained, and the number of events was supposed to be no less than 10 times the predictors.
Finally, sex, smoking status, Karnofsky Performance Status, TTF-1, and EGFR mutation were selected as the predictors for survival status of lung adenocarcinoma. The C-index and 95% CI of the nomogram was 0.705 (0.678–0.798). The calibration curve showed a good efficiency (Figure 3), demonstrating that our predicted survival chances were indeed consistent with actual observations.
TTF-1 predictive impact: benefit to first-line pemetrexed-based chemotherapy
In order to determine the predictive impact of TTF-1, we evaluated the duration of first-line pemetrexed-based chemotherapy rather than progression-free survival. We analyzed the data of TTF-1 expression and non-TTF-1 expression patients respectively in the interest of avoiding other confounding factor affected the survival. We hypothesized that only patients with a positive TTF-1 expression would have the benefit of the initial pemetrexed-based chemotherapy. However, the duration of treatment was improved in TTF-1-positive and TTF-1-negative patients who received pemetrexed basic chemotherapy compared with those who received non-pemetrexed chemotherapy (among TTF-1 positive patients: HR =0.34, 95% CI: 0.20–0.57, P<0.0001, Table 3; among TTF-1 negative patients: HR =0.69, 95% CI: 0.30–1.60, P=0.39, Table 3).

Full table
Discussion
To clarify the prognostic influence of TTF-1, we collected a series of consecutive patients with stage IV lung adenocarcinoma in China. We found that patients with TTF-1 positive had a better survival than the patients with TTF-1 negative (median overall survival time of 22.7 and 11.8 months respectively, P<0.01 except the subgroup of patients with EGFR mutation or received targeted therapy). The lack of statistical significance may be due to the limited number of patients with TTF-1 negative TTF-1 mutations (only 7 of 60 patients with identified EGFR mutations are TTF-1 negative), which limits this comparison’s performance. The conclusion that TTF-1 is robust prognostic marker is further strengthened by the increased overall survival in almost all subgroups in multivariate analysis of clinical and treatment characteristics. The prognostic influence of TTF-1 is the same as, or even exceeds other variables examined in our study, including age, performance state, smoking history, receiving doublet or triplet chemotherapy or targeted therapy. Our study suggests that TTF-1 was an independent, far-reaching significant prognostic variable in advanced lung adenocarcinomas among non-Asian patients.
TTF-1 has been researched as a potential prognostic indicator in NSCLC presenting inconsistent results. One study (15) demonstrated that TTF-1 expression is associated with worse survival. Several investigations have reported no relationship between TTF-1 expression and lung adenocarcinomas (16-21). However, some studies showed a positive influence of TTF-1 on survival (22-30). The reason TTF-1’s prognostic effect seems to be underused may be due to relatively small sample size or lack of control over histology and staging in previous studies. Barletta et al. (26), for example, found that TTF-1 expression was linked to better survival, but the effect of the disease stage on the association between TTF-1 expression and overall survival did not enter the decision. A larger cohort study suggested TTF-1 expression has a positive effect on the survival of patients with stage I pulmonary adenocarcinoma (11). The prognostic effects of TTF1 in advanced NSCLC were previously tested in two small studies showing a strong prognostic benefit of TTF1-positive (25,31). A recently published meta-analysis indicated that TTF-1 overexpression is associated with a favorable prognosis in patients with NSCLC, particularly in patients with stage I and stage IIIb–IV NSCLC and lung AC (32). Except for evaluating the prognostic impact of TTF-1 on survival between advanced lung adenocarcinomas, we also examined whether TTF-1 positive lung adenocarcinoma patients will benefit more from pemetrexed-based chemotherapy than TTF-1 negative patients. The findings of TTF1 expression showed by Sun et al. (33) to be substantially related to improved clinical results in NSCLC-treated non-squamous patients with pemetrexed therapy. However patients who had not been exposed to pemetrexed were not analyzed, and histology was not limited strictly. Different from previous studies, we used the duration of treatment on initial pemetrexed-based chemotherapy instead of progression-free survival in our study. We found that both TTF-1 positive and negative patients receiving pemetrexed-based chemotherapy could be improved in the duration of treatment. A phase III study demonstrated that patients with advanced-stage NSCLC could benefit from chemotherapy based on cisplatin/pemetrexed. Our results are consistent with previous study on non-Asian population and provided that TTF-1 expression does not forecast the apparent benefit of pemetrexed chemotherapy.
However, there are many limitations to our research. First of all, the way we conducted research as a retrospective study. Based on the retrospective nature, we have instead used the duration of treatment instead of the progression-free survival to avoid other confounding factors which had affected the survival rate and to compensate for the retrospective analysis. Additionally, the data was drawn from a single institute, and various potential biases could not be eliminated, such as the amount of metastatic lesions. But we are the first one to study whether the expression of TTF-1 can further predict the lung adenocarcinoma subgroup sensitive to pemetrexed in a Chinese population.
In conclusion, TTF-1 expression was significantly associated with a better clinical outcome for the patients with advanced lung adenocarcinomas. Therefore, TTF-1 expression in tumor tissues might be a helpful prognostic factor. Further research is needed to explore the physiologic mechanisms underlying the effects of TTF-1 on the biology of lung cancers. We found that TTF-1 expression does not forecast an apparent profit from pemetrexed chemotherapy. Furthermore, the predictive role of TTF-1 expression for pemetrexed-based chemotherapy ought to be confirmed in a prospective and randomized study.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Affiliated Hospital of Qingdao University (No. QYFY WZLL 25595) approved this retrospective biomarker study.
Data Sharing Statement: No additional data available.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630-5. [Crossref] [PubMed]
- Kian W, Roisman LC, Peled N. Two are better than one on progression through MET mechanism for EGFR+ NSCLC patients. Transl Lung Cancer Res 2018;7:S334-5. [Crossref] [PubMed]
- Gkolfinopoulos S, Mountzios G. Beyond EGFR and ALK: targeting rare mutations in advanced non-small cell lung cancer. Ann Transl Med 2018;6:142. [Crossref] [PubMed]
- Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642-50. [Crossref] [PubMed]
- Tanzawa S, Ishihara M, Haruyama T, et al. Which is better, EGFR-TKI mono or combination for non-small cell lung cancer with mutated EGFR? Transl Cancer Res 2019;8:2223-9. [Crossref]
- Fujita J, Ohtsuki Y, Bandoh S, et al. Expression of thyroid transcription factor-1 in 16 human lung cancer cell lines. Lung Cancer 2003;39:31-6. [Crossref] [PubMed]
- Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol 2011;24:1348-59. [Crossref] [PubMed]
- Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007;450:893-8. [Crossref] [PubMed]
- Maeda Y, Tsuchiya T, Hao H, et al. Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest 2012;122:4388-400. [Crossref] [PubMed]
- Yamaguchi T, Yanagisawa K, Sugiyama R, et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell 2012;21:348-61. [Crossref] [PubMed]
- Anagnostou VK, Syrigos KN, Bepler G, et al. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol 2009;27:271-8. [Crossref] [PubMed]
- Chen YF, Hsieh MS, Wu SG, et al. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J Thorac Oncol 2016;11:1140-52. [Crossref] [PubMed]
- Drilon A, Bergagnini I, Delasos L, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol 2016;27:1286-91. [Crossref] [PubMed]
- Schilsky JB, Ni A, Ahn L, et al. Prognostic impact of TTF-1 expression in patients with stage IV lung adenocarcinomas. Lung cancer 2017;108:205-11. [Crossref] [PubMed]
- Lee JS, Kim HR, Lee CY, et al. EGFR and TTF-1 gene amplification in surgically resected lung adenocarcinomas: clinicopathologic significance and effect on response to EGFR-tyrosine kinase inhibitors in recurred cases. Ann Surg Oncol 2013;20:3015-22. [Crossref] [PubMed]
- Pelosi G, Fraggetta F, Pasini F, et al. Immunoreactivity for thyroid transcription factor-1 in stage I non-small cell carcinomas of the lung. Am J Surg Pathol 2001;25:363-72. [Crossref] [PubMed]
- Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korean Med Sci 2003;18:494-500. [Crossref] [PubMed]
- Au NH, Cheang M, Huntsman DG, et al. Evaluation of immunohistochemical markers in non-small cell lung cancer by unsupervised hierarchical clustering analysis: a tissue microarray study of 284 cases and 18 markers. J Pathol 2004;204:101-9. [Crossref] [PubMed]
- Stenhouse G, Fyfe N, King G, et al. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 2004;57:383-7. [Crossref] [PubMed]
- Berghmans T, Mascaux C, Martin B, et al. Prognostic role of thyroid transcription factor-1 in stage III non-small cell lung cancer. Lung cancer 2006;52:219-24. [Crossref] [PubMed]
- Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol 2009;4:951-8. [Crossref] [PubMed]
- Haque AK, Syed S, Lele SM, et al. Immunohistochemical study of thyroid transcription factor-1 and HER2/neu in non-small cell lung cancer: strong thyroid transcription factor-1 expression predicts better survival. Appl Immunohistochem Mol Morphol 2002;10:103-9. [Crossref] [PubMed]
- Tan D, Li Q, Deeb G, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol 2003;34:597-604. [Crossref] [PubMed]
- Saad RS, Liu YL, Han H, et al. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol 2004;35:3-7. [Crossref] [PubMed]
- Barlési F, Pinot D, Legoffic A, et al. Positive thyroid transcription factor 1 staining strongly correlates with survival of patients with adenocarcinoma of the lung. Br J Cancer 2005;93:450-2. [Crossref] [PubMed]
- Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med 2009;13:1977-86. [Crossref] [PubMed]
- Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res 2011;17:2434-43. [Crossref] [PubMed]
- Li X, Wan L, Shen H, et al. Thyroid transcription factor-1 amplification and expressions in lung adenocarcinoma tissues and pleural effusions predict patient survival and prognosis. J Thorac Oncol 2012;7:76-84. [Crossref] [PubMed]
- Solis LM, Behrens C, Raso MG, et al. Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer 2012;118:2889-99. [Crossref] [PubMed]
- Chung KP, Huang YT, Chang YL, et al. Clinical significance of thyroid transcription factor-1 in advanced lung adenocarcinoma under epidermal growth factor receptor tyrosine kinase inhibitor treatment. Chest 2012;141:420-8. [Crossref] [PubMed]
- Martins SJ, Takagaki TY, Silva AG, et al. Prognostic relevance of TTF-1 and MMP-9 expression in advanced lung adenocarcinoma. Lung Cancer 2009;64:105-9. [Crossref] [PubMed]
- Qian HH, Xu TS, Cai XQ, et al. Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: A meta-analysis. Clin Chim Acta 2015;451:208-14. [Crossref] [PubMed]
- Sun JM, Han J, Ahn JS, et al. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol 2011;6:1392-9. [Crossref] [PubMed]