
Significance of tumor spread through air spaces (STAS) in lung cancer from the pathologist perspective
Introduction
Invasion in lung cancer is traditionally defined as: (I) a non-lepidic histologic pattern of growth; (II) myofibroblastic proliferation with desmoplasia; and (III) vascular or pleural invasion. The 2015 World Health Organization (WHO) Classification of Lung Tumors fascicle has introduced a new concept of invasion, spread through air spaces (STAS) that is defined as “spread of micropapillary clusters, solid nests, and/or single cancer cells into airspaces in the lung parenchyma beyond the edge of the main tumor.” (1,2). It is assumed that STAS represents airspace invasion that is unique to the lung, and may be considered equal to other more established patterns of invasive growth, such as lymphovascular or pleural invasion.
The significance of this concept is accentuated given the updated lung adenocarcinoma classification system outlining criteria for in-situ, minimally invasive, and invasive adenocarcinoma categories as proposed by multidisciplinary panels from the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) and adopted by the 8th edition of the American Joint Committee on Cancer (AJCC) (3,4). Now, the presence of STAS excludes a diagnosis of adenocarcinoma in-situ or minimally invasive adenocarcinoma in small tumors.
Further, sublobar resections (wedge resection and segmentectomy) have been increasingly applied for not only poor-risk patients but also good-risk patients with clinical stage IA non-small cell lung cancer (NSCLC), given the increase in detection of small peripheral tumors secondary to the advancement of imaging techniques (5-8). The presence of STAS in sublobar resection specimens is now considered an indication for completion lobectomy by some surgeons. Thus, it is important for pathologists to recognize and report STAS.
In this review, I will discuss the evolution of our understanding of airspace invasion over the past decade and the current concept, controversies and practical issues associated with STAS from the pathologist perspective.
Concept of airspace invasion
The concept of airspace invasion is not new. Kodama and colleagues reported in 1980 a case with multifocal “aerogenous” spread that appeared to be equivalent to STAS based on its description (9). They showed using electron microscopy that the tumor cells in airspaces were rather poorly differentiated but somewhat resembled the hyperplastic cuboidal alveolar cells seen in the damaged lung and that they proliferated in airways, representing “aerogenous metastases.” Further, the authors concluded that biologic behavior of the tumor cells might be partly explained by their dyshesive nature (9). Subsequently, Chung and colleagues at Seoul National University Bundang Hospital implemented aerogenous spread in their lung cancer synoptic report in 2008 (personal communication). Yi and colleagues also reported the presence of aerogenous spread as an independent pathological risk factor of recurrence in stage I lung adenocarcinoma by multivariate analysis (10).
Similarly, Onozato and colleagues found unique island-like structures in lung adenocarcinomas by 3D reconstruction and named them as “tumor islands” (11). Tumor island is a detached, large cluster of tumor cells in an alveolar space separated from the main tumor mass, but has been confirmed to be connected to the main tumor in different tissue levels by the 3D reconstruction. Tumor islands appear to share characteristic features with some of the solid nest type of STAS, in particular large ones (see below). The subsequent clinicopathologic study revealed their association with aggressive pathologic features, KRAS mutations and unfavourable prognosis in 261 stage I-II lung adenocarcinomas (12).
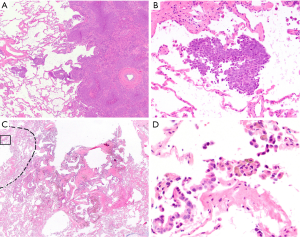
Subsequently, Kadota and colleagues proposed the current form of airspace invasion “tumor spread through airspaces” (STAS) and designated the possibility of airspace invasion as a new pattern of invasion in 2015 (13). They defined STAS as the spread of tumor cells (as micropapillary structures, solid nests, and/or single cells) within airspaces in the lung parenchyma beyond the edge of the main tumor, even if it existed only in the first alveolar layer from the tumor edge (Figure 1), and found STAS in 38% of 144 resected, small stage I lung adenocarcinomas. Upon correlating STAS with clinicopathologic features, STAS-positive tumors were more likely to show, lymphovascular invasion, vascular invasion, micropapillary pattern, solid pattern and less lepidic pattern in the main tumor, and the presence of STAS was associated with an increased risk of any types of recurrence in sublobar resections. Further, STAS was an independent and the only risk factor of any recurrence (HR, 3.08; P=0.014) in multivariate analysis in the sublobar resection cohort, while STAS was not associated with an increased risk of any recurrence [5-year cumulative incidence of recurrence (CIR), 12.7% vs. 9.5%; P=0.50] in the lobectomy cohort. The overall findings suggest that the presence of STAS indicates a higher risk of recurrence in small lung adenocarcinomas and that limited resection may not be sufficient for those with STAS (13).
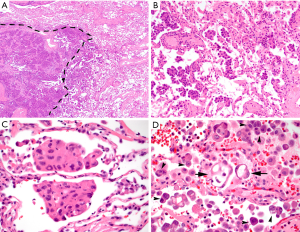
STAS and its prognostic implications in lung cancer
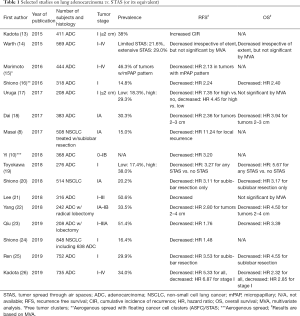
Since the original report of STAS, there have been multiple studies that reported the prevalence and prognostic significance of STAS and its equivalent mostly in lung adenocarcinoma (Table 1) (8,10,13-26). Although the majority of reported studies have focused on early stage lung adenocarcinoma, similar findings have also been demonstrated in late stage adenocarcinoma. For instance, Warth and colleagues showed that the presence of STAS had significantly reduced recurrence free survival (RFS) and overall survival (OS) at any stage, and is more commonly seen in high stage, node positive adenocarcinomas with distant metastases (14). Another study by Terada and colleagues showed that STAS was a significant risk factor for recurrence even in stage III adenocarcinoma (27). Further, Dai and colleagues showed that stage IA adenocarcinoma with STAS had RFS and OS comparable to stage IB adenocarcinoma, suggesting not only the aggressive biology but also the implications of STAS on the future of T staging (18).
Full table
The reported frequency of STAS in NSCLC ranges from 15–56% depending on tumor stages included in the cohort (Table 1), and the vast majority of the studies showed association of STAS with micropapillary and/or solid patterns and less lepidic pattern in the main tumor and other adverse pathologic features. Several studies also reported higher rates of KRAS or BRAF mutations, ALK or ROS1 rearrangements and wild-type EGFR in lung adenocarcinomas with STAS (12,14,21,26,28). Importantly, almost all studies have confirmed the association of STAS with shorter RFS and/or shorter OS. Consequently, STAS was included as an exclusion criterion along with lymphovascular and pleural invasions for adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) in WHO 2015 (1).
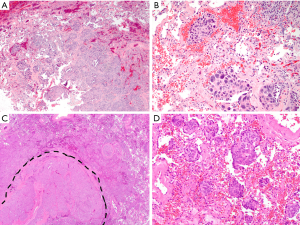
STAS has also been described in other primary lung malignancies, including squamous cell carcinoma (SCC), neuroendocrine tumors and pleomorphic carcinoma (Figure 2). STAS in primary lung SCC was first characterized by Lu and colleagues in a study of 445 patients, in which the presence of STAS was associated with significantly higher cumulative incidence of relapse (CIR). Interestingly, however, there was no difference in overall survival (29). An additional study by Kadota and colleagues confirmed that STAS was an independent predictor of RFS in all stages of SCC (30); however, another study has suggested that it is a useful predictor only in stage I tumors (31). There is also recent literature on primary lung neuroendocrine neoplasms (32-34). Of those, Aly and colleagues retrospectively studied 487 neuroendocrine tumors, including typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma (LCNEC), and small cell carcinoma (SCLC) (33). Histologic review showed that 26% of these patients had evidence of STAS, which was associated with distant metastasis and a higher CIR and lung cancer-specific cumulative incidence of death (LC-CID) overall (independent of histologic subtype). Further, they found STAS in LCNEC and SCNEC to be an independent poor prognostic factor, while Toyokawa and colleagues reported high (83%) frequency of STAS identified in SCLC but no significant differences in RFS and OS between patients with no/low STAS and those with high STAS (32).
Recently, the first systematic reviews and meta-analyses of STAS were published, and both confirmed that the presence of STAS was associated with shorter RFS and OS with an absent to moderate heterogeneity between studies (35,36). Subgroup analysis by tumor histology revealed that STAS associated with adenocarcinoma had shorter OS, but interestingly, STAS associated with SCC or pleomorphic carcinoma did not demonstrate a shorter OS, attributed in part to the older age of the patients (29,35). Nevertheless, it has become clear that STAS is a very important prognostic factor across lung adenocarcinoma of all stages (Table 1), and is likely important in other histologic subtypes of lung carcinoma as well.
Clinical implications of STAS in stage IA NSCLC
Lobectomy has been the standard surgical procedure for clinical stage IA NSCLC (37); however, sublobar resection, including wedge resection and segmentectomy, is considered an acceptable alternate for comorbid patients to preserve pulmonary function (38). Further, the recent advancement in imaging studies has resulted in increased detection of early stage lung cancers, either incidental or thorough low-dose CT lung screening (38-40). Subsequently, although the evidence is still insufficient, limited resection has increasingly been applied for good-risk patients with early stage NSCLC as well as for poor-risk patients (5-7).
Importantly, a recent large cohort study by Eguchi and colleagues has shown that sublobar resection is suboptimal for T1 lung adenocarcinoma with STAS from the oncology perspective (41). They evaluated multiple clinicopathologic variables including STAS in propensity score-matched lobectomy (n=349) and sublobar resection (n=349) cohorts and reported that the presence of STAS was associated with higher rates of recurrence and cancer-specific death in the sublobar resection cohort but not in the lobectomy cohort, irrespective of surgical margin clearance in patients with STAS. The findings are in line with those of the initial study by Kadota and colleagues (13) and more recent literature in early stage tumors (13,20,41). However, other studies have not found a survival difference between sublobar resections and lobectomies (15,19,20,42).
Nevertheless, some surgeons recommend that the patient with small T1N0M0 adenocarcinoma undergo subsequent completion lobectomy when STAS is found in a sublobar resection specimen (personal experience and communication with thoracic surgeons at a few US academic centers). This clinical scenario is not extremely uncommon given that sublobar resection has been increasingly applied for patients with small, node negative lung adenocarcinoma, and tumor size (≤2 vs. >2 cm) plays a significant role in preoperative decision making on the type of resection (sublobar resection vs. lobectomy) to perform (6).
In the aforementioned study, Eguchi and colleagues reported an optimal performance of frozen section in identifying STAS with the overall sensitivity of 71%, specificity of 92%, overall agreement of 75% and substantial interobserver concordance (41). The findings imply that FS analysis may be useful to detect STAS and aid intraoperative decision making on the most appropriate type of resection for a patient with early-stage lung adenocarcinoma. Thus, thoracic surgeons in many academic institutions (at least in the US) now request the pathologist to evaluate the presence or absence of STAS (along with histology, resection margin status, etc.) at the time of intraoperative consultation. This has made the pathologist realize significant clinical implications of the STAS diagnosis including that on frozen section. However, to implement STAS in routine diagnosis/lung cancer synoptic report as well as frozen section diagnosis, there are multiple issues to address. First, there have been controversies as to whether STAS is a real biological phenomenon or merely represents ex vivo artifacts. In fact, some pathologists do not believe in the biological relevance of STAS; thus, they do not diagnose it. Second, the definition of STAS has not been established, while it is important to have the universally accepted gold standard to achieve adequate interobserver concordance. Third, only limited and conflicting data are available to assess the performance of FS on the diagnosis of STAS.
Controversies on STAS vs. ex vivo artifacts
There have been controversies as to whether any or all of the free-floating tumor cell clusters identified as STAS are actually ex vivo artifactual. There are multiple potential mechanisms that may lead to the development of free floating tumor cells ex vivo that complicate interpretation of STAS (43,44). Some of these are routine artifacts specific to lung specimens, including surgical collapse, but the most notable is the possibility that the tumor cell clusters can be spread through knife cuts made at the time of specimen processing. The concept of spread through a knife surface (STAKS) was first introduced by Thunnissen and colleagues, who showed that artifactual carryover after a knife cut is a real phenomenon that increases free-floating tumor clusters with each sequential cut, if the knife is not cleaned between the cuts (44). Subsequently, Blaauwgeers and colleagues have shown in their multi-institutional study that the number of free-floating tumor cell clusters increased in sequential sections with the same prosecting blade and that the vast majority of the free-floating tumor cell clusters could be explained by mechanical forces associated with tissue handling (43).
However, there are multiple conditions, not only ex vivo but also in vivo, that could generate mechanical forces leading to the detachment of tumor cell clusters including palpation by the surgeon at the time of operation and retrieval of the specimen through a small caliber of VATS port as well as knife cuts during tissue processing. Even high flow velocities inherent to the physics of breathing may lend themselves to the detachment of tumor cell clusters, and once the clusters appear in airspaces beyond the border of the tumor, they are considered free-floating tumor clusters (STAS or “artifacts”). Interestingly, Isaka and colleagues detected tumor cell clusters in one fifth of airway secretion cytology specimens collected from the segmental or lobar bronchus of resected lung adenocarcinomas and squamous cell carcinomas. Further, the positive airway secretion cytology was only seen in tumors with histologically confirmed STAS, and the morphology of STAS was similar to that of the tumor cell clusters in the airway secretion (45). Ren and colleagues also identified isolated tumor cell lusters in the residual lung parenchyma in 9% of simulated segmentectomy specimens that were made from lobectomies for stage 1A adenocarcinoma (25). These findings support the notion that free-floating tumor cell clusters could be generated by palpation by the surgeon at the time of operation.
Given the fact that air spaces are surrounded by a capillary network in the alveolar interstitium in the normal lung, the free-floating clusters, if they remain in the residual lung parenchyma, could survive and may become a new nidus for neoplastic proliferation/tumor recurrence. In general, STAS may be considered akin to the findings in invasive mucinous adenocarcinoma in which the presence of abundant extracellular mucin serves as a fluid conduit for the spread of neoplastic cells to more distant locales (1). Although the tumor cells of STAS do not produce the medium for spread by themselves, it is conceivable that these free-floating tumor clusters can also remain viable in the alveolar space for extended periods of time, given that alveolar macrophages can survive within air spaces for up to 40 days (46). Further, a recent study using multiplex immunofluorescence has shown tumor clusters attached to alveolar walls away from the main tumor and close proximity to an alveolar capillary (vessel co-option) (47). We have also experienced cases demonstrating re-attachment of tumor clusters to normal alveolar walls away from the main tumor with associated stromal reaction (Figure 3).
Now, it is reasonable to think that the differentiation of free-floating tumor cell clusters that developed before resection of the tumor (in vivo) and those generated after resection (ex vivo) would have significant clinical implications. However, in the opinion of the author, the question of how much of this morphology might represent artifact and when it is generated is a somewhat academic one. The fact remains that multiple studies (that made reasonable efforts to differentiate STAS from obvious processing artifacts) have shown that the presence of STAS can be used at least as a surrogate for aggressive behavior and has often been identified as an independent prognostic factor (Table 1). The prognostic significance would be difficult, if not impossible, to explain by artifact alone. Further, given the association of STAS and micropapillary or high-grade patterns in the main tumor, the possibility that the processing artifacts may be related to the inherent discohesiveness (“invasiveness”) of the tumor cells.
Differentiation from ex vivo artifacts
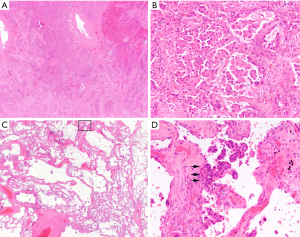
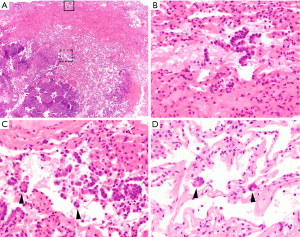
Now one of the most important issues associated with STAS for the pathologist is to differentiate STAS from “real” artifacts made by strenuous mechanical forces generated by knife cuts during tissue processing. In order to help the differentiation, Kadota (13) and Aly (33) have described several features of artifacts as exclusion criteria of STAS: (I) mechanically dissociated tumor floaters (clusters of tumor cells with a ragged-edge, located randomly and/or located at the edge of the tumor section) (Figure 4A,B); (II) normal benign pneumocytes or bronchial cells (Figure 4C,D); (III) strips of tumor cells detached from alveolar walls or stroma due to poor preservation; (IV) isolated tumor clusters distantly situated away from the tumor not in a continuous manner. They also considered the presence of a single focus of STAS in the entire tumor as an artifact. Additionally, Warth and colleagues emphasized the presence of loose small groups and distribution consistent with the overall configuration of the circumferential tumor edge in differentiating STAS from artifacts (14). STAS may occasionally be somewhat difficult to distinguish from intra-alveolar macrophages, and the distinction should be made on the bases of nuclear-to-cytoplasmic ratio and degree of nuclear atypia (Figure 1D). In difficult cases, an immunohistochemical stain for cytokeratin and/or that for CD68/CD163 may be helpful to make the distinction. Similarly, detached fragments of benign respiratory epithelium may exhibit reactive atypia and mimic tumor cells. They may be confused with STAS, in particular, at the time of frozen section (Figure 4C,D). The linear configuration is a useful feature for differentiation of an artifact from STAS in this context.
Definition of STAS
The evaluation of STAS in the literature has been somewhat limited by some variability in definitions that have been applied. While the majority of studies have adopted the original description by Kadota and colleagues that defined STAS as micropapillary clusters, solid nests, and/or single cells within airspaces in the lung parenchyma beyond the edge of the main tumor (13), minimum number of tumor cells and/or clusters and the necessary distance from the border of the main tumor have not been so well-established. For instance, various studies have used distances from the first alveolar layer beyond the tumor edge, to a few alveolar spaces, to at least 3 mm from the main tumor (Table 2) (8,10,13-22,24-26,28). Importantly, however, the variation in definition has not hampered agreement across multiple studies that there is an association between STAS and decreased RFS and/or OS (Table 1).
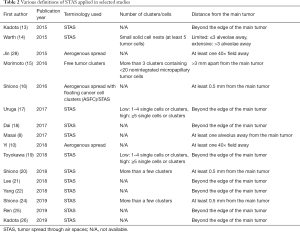
Full table
In contrast, the amount of STAS appears to be significant. Recent studies by Uruga and colleagues and Toyokawa and colleagues used a semi-quantitative approach to assess the amount of STAS in the average ×200 microscopic field as no STAS; low STAS (1–4 tumor cell clusters in tumors with micropapillary or solid STAS, or 1–4 tumor cells in single-cell predominant STAS); and high STAS (≥5 tumor cell clusters in tumors with micropapillary or solid STAS, or ≥5 tumor cells in single-cell predominant STAS) (17,19). Both studies found low STAS in 17–18% and high STAS in 29–38% of stage 1 lung adenocarcinomas. Uruga and colleagues also reported the association of high STAS with pleural invasion, lymphovascular invasion, and a solid-predominant pattern of growth, while both showed a significant association between increased STAS and shorter RFS by univariate analysis, and STAS being a significant predictor of RFS by multivariate analysis (17,19).
There is only limited data on interobserver concordance for the diagnosis of STAS; however, the globally accepted definition of STAS, if established, would improve interobserver agreement on STAS and facilitate the implementation of STAS diagnosis in routine pathology practice.
Performance of frozen section on the diagnosis of STAS
While the aforementioned study by Eguchi indicates the potential utility of frozen section for the intraoperative assessment of STAS, given its high sensitivity, excellent specificity and substantial interobserver concordance on STAS diagnosis (41), Walts and colleagues reported rather discouraging results. In their study with 48 stage I-II lung adenocarcinoma resections, they found 50% sensitivity and only 8% negative predictive value of frozen section in identifying STAS, while its positive predictive value was 100% (48). They suggest that the selection of samples including a larger portion of nonneoplastic tissue adjacent to the tumor and/or microscopic examination of additional deeper levels prepared from the frozen tissue block are important in a confident designation of STAS. These processes are not typically feasible at the time of frozen section, however. Further, Morimoto and colleagues clearly state that the diagnosis of STAS may only be possible on permanent section since, in frozen section, the lung is not sufficiently inflated to optimally assess detached tumor cells/clusters in airspaces (15). In our experience, frozen section is highly specific but not sensitive for the diagnosis of STAS in early stage lung adenocarcinoma, with only fair interobserver agreement at frozen section. We also found frequent processing artifacts in frozen section (and frozen permanent) slides with some along with STAS, possibly leading to the low sensitivity and fair interobserver agreement of the STAS diagnosis (Figure 5) (unpublished data). Disagreement in the findings in current literature regarding the ability of pathologists to confidently identify STAS suggests interinstitutional variation in its interpretation. While it appears premature to globally implement the assessment of STAS at frozen section, pathologists may elect to comment on the presence of unequivocal STAS when seen at frozen section. It is important, however; thoracic surgeons should be advised that there has been no prospective data to confirm that this information is useful to help stratify patients for lobectomy versus sublobar resections intraoperatively (48).
Conclusion
Irrespective of how the detached tumor cells/clusters are generated, STAS is biologically important and is associated with unfavorable patient outcomes in not only lung adenocarcinomas but also in other types of lung cancer. STAS has significant clinical implications; thus, we, pathologists, need to recognize and appropriately report STAS. Therefore, establishment of the globally accepted definition, confident differentiation from processing artifacts and standardization in reporting of STAS including at the time of intraoperative consultation are warranted.
Acknowledgments
The author thanks Mr. Stephen A. Conley with editing images for publication and Mr. Cris Kenudson and Ms. Mayerling R. Dada for their editorial assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.01.06). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. MMK serves as the unpaid editorial board member of Translational Lung Cancer Research from Jul. 2019 to Jul. 2021. MMK has served as a compensated consultant for H3 Biomedicine and AstraZeneca; reports personal fees from H3 Biomedicine, personal fees from AstraZeneca, grants from Novartis, outside the submitted work.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer; 2015.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Amin MB ES, Greene F, Byrd DR, et al. AJCC Cancer Staging Manual. 8th ed: Springer International Publishing; 2017.
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Brown LM, Louie BE, Jackson N, et al. Recurrence and Survival After Segmentectomy in Patients With Prior Lung Resection for Early-Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1110-8. [Crossref] [PubMed]
- Takahashi Y, Kuroda H, Oya Y, et al. Challenges for real-time intraoperative diagnosis of high risk histology in lung adenocarcinoma: A necessity for sublobar resection. Thorac Cancer 2019;10:1663-8. [Crossref] [PubMed]
- Madariaga ML, Lennes IT, Best T, et al. Multidisciplinary selection of pulmonary nodules for surgical resection: Diagnostic results and long-term outcomes. J Thorac Cardiovasc Surg 2020;159:1558-66.e3. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Kodama T, Kameya T, Shimosato Y, et al. Cell incohesiveness and pattern of extension in a rare case of bronchioloalveolar carcinoma. Ultrastruct Pathol 1980;1:177-88. [Crossref] [PubMed]
- Yi E, Bae MK, Cho S, et al. Pathological prognostic factors of recurrence in early stage lung adenocarcinoma. ANZ J Surg 2018;88:327-31. [Crossref] [PubMed]
- Onozato ML, Klepeis VE, Yagi Y, et al. A role of three-dimensional (3D)-reconstruction in the classification of lung adenocarcinoma. Anal Cell Pathol (Amst) 2012;35:79-84. [Crossref] [PubMed]
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016;152:64-72.e1. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Uruga H, Fujii T, Fujimori S, et al. Semiquantitative Assessment of Tumor Spread through Air Spaces (STAS) in Early-Stage Lung Adenocarcinomas. J Thorac Oncol 2017;12:1046-51. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Pathological Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:1655-63. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread Through Air Spaces Is a Prognostic Factor in Sublobar Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Lee JS, Kim EK, Kim M, et al. Genetic and clinicopathologic characteristics of lung adenocarcinoma with tumor spread through air spaces. Lung Cancer 2018;123:121-6. [Crossref] [PubMed]
- Yang L, Yang Y, Ma P, et al. Spread through air spaces predicts a worse survival in patients with stage I adenocarcinomas >2 cm after radical lobectomy. J Thorac Dis 2018;10:5308-17. [Crossref] [PubMed]
- Qiu X, Chen D, Liu Y, et al. Relationship between stromal cells and tumor spread through air spaces in lung adenocarcinoma. Thorac Cancer 2019;10:256-67. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread through air spaces in lung cancer patients is a risk factor for pulmonary metastasis after surgery. J Thorac Dis 2019;11:177-87. [Crossref] [PubMed]
- Ren Y, Xie H, Dai C, et al. Prognostic Impact of Tumor Spread Through Air Spaces in Sublobar Resection for 1A Lung Adenocarcinoma Patients. Ann Surg Oncol 2019;26:1901-8. [Crossref] [PubMed]
- Kadota K, Kushida Y, Kagawa S, et al. Limited Resection Is Associated With a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am J Surg Pathol 2019;43:1033-41. [Crossref] [PubMed]
- Terada Y, Takahashi T, Morita S, et al. Spread through air spaces is an independent predictor of recurrence in stage III (N2) lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2019;29:442-8. [Crossref] [PubMed]
- Jin Y, Sun PL, Park SY, et al. Frequent aerogenous spread with decreased E-cadherin expression of ROS1-rearranged lung cancer predicts poor disease-free survival. Lung Cancer 2015;89:343-9. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Endo M, et al. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer 2018;120:14-21. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. High Frequency of Spread Through Air Spaces in Resected Small Cell Lung Cancer. Anticancer Res 2018;38:1821-5. [PubMed]
- Aly RG, Rekhtman N, Li X, et al. Spread Through Air Spaces (STAS) Is Prognostic in Atypical Carcinoid, Large Cell Neuroendocrine Carcinoma, and Small Cell Carcinoma of the Lung. J Thorac Oncol 2019;14:1583-93. [Crossref] [PubMed]
- Altinay S, Metovic J, Massa F, et al. Spread through air spaces (STAS) is a predictor of poor outcome in atypical carcinoids of the lung. Virchows Arch 2019;475:325-34. [Crossref] [PubMed]
- Chen D, Mao Y, Wen J, et al. Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2019;108:945-54. [Crossref] [PubMed]
- Liu A, Hou F, Qin Y, et al. Predictive value of a prognostic model based on pathologic features in lung invasive adenocarcinoma. Lung Cancer 2019;131:14-22. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Uruga H, Fujii T, Miyamoto A, et al. What did the first meta-analysis of tumor spread through air spaces (STAS) bring to light? J Thorac Dis 2019;11:S1979-81. [Crossref] [PubMed]
- Blaauwgeers H, Flieder D, Warth A, et al. A Prospective Study of Loose Tissue Fragments in Non-Small Cell Lung Cancer Resection Specimens: An Alternative View to "Spread Through Air Spaces". Am J Surg Pathol 2017;41:1226-30. [Crossref] [PubMed]
- Thunnissen E, Blaauwgeers HJ, de Cuba EM, et al. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch Pathol Lab Med 2016;140:212-20. [Crossref] [PubMed]
- Isaka T, Yokose T, Miyagi Y, et al. Detection of tumor spread through airspaces by airway secretion cytology from resected lung cancer specimens. Pathol Int 2017;67:487-94. [Crossref] [PubMed]
- Murphy J, Summer R, Wilson AA, et al. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:380-5. [Crossref] [PubMed]
- Yagi Y, Aly RG, Tabata K, et al. Three-Dimensional Histologic, Immunohistochemical and Multiplex Immunofluorescence Analysis of Dynamic Vessel Co-Option of Spread through Air Spaces (Stas) in Lung Adenocarcinoma. J Thorac Oncol 2019. In press. [PubMed]
- Walts AE, Marchevsky AM. Current Evidence Does Not Warrant Frozen Section Evaluation for the Presence of Tumor Spread Through Alveolar Spaces. Arch Pathol Lab Med 2018;142:59-63. [Crossref] [PubMed]


